News & Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Prescription Drugs Requiring Prior Authorization
PRESCRIPTION DRUGS REQUIRING PRIOR AUTHORIZATION Revised 10/16 As part of our drug utilization management program, members must request and receive prior authorization for certain prescription drugs in order to use their prescription drug benefits. Below is a list of drugs that currently require prior authorization. This list will be updated periodically as new drugs that require prior authorization are introduced. As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. The Schedule of Benefits contains a list of drug categories that require prior authorization. Prior authorization requests are processed by our pharmacy benefit manager, Express Scripts, Inc. (ESI). Physicians must call ESI to obtain an authorization. (1-800-842-2015). Drug Name Generic Name Drug Classification Abstral fentanyl citrate oral tablet Controlled Dangerous Substance Accu-Chek Test Strips blood glucose test strips Blood Glucose Test Strips Actemra tocilizumab Monoclonal Antibody Acthar corticotropin Hormone Actimmune interferon gamma 1b Interferon Actiq fentanyl citrate OTFC Controlled Dangerous Substance Adcirca tadalafil Pulmonary Vasodilator Adempas riociguat Pulmonary Vasodilator Adlyxin lixisenatide Type 2 Diabetes Advocate Test Strips blood glucose test strips Blood Glucose Test Strips Aerospan** flunisolide Corticosteroids (Inhaled) Afrezza insulin Insulin (inhaled) Ampyra dalfampridine Multiple Sclerosis Agent Altoprev** lovastatin Cholesterol Alvesco** ciclesonide Corticosteroids -

Circulating Biomarkers in Neuromuscular Disorders: What Is Known, What Is New
biomolecules Review Circulating Biomarkers in Neuromuscular Disorders: What Is Known, What Is New Andrea Barp 1,* , Amanda Ferrero 1, Silvia Casagrande 1,2 , Roberta Morini 1 and Riccardo Zuccarino 1 1 NeuroMuscular Omnicentre (NeMO) Trento, Villa Rosa Hospital, Via Spolverine 84, 38057 Pergine Valsugana, Italy; [email protected] (A.F.); [email protected] (S.C.); [email protected] (R.M.); [email protected] (R.Z.) 2 Department of Neurosciences, Drug and Child Health, University of Florence, Largo Brambilla 3, 50134 Florence, Italy * Correspondence: [email protected] Abstract: The urgent need for new therapies for some devastating neuromuscular diseases (NMDs), such as Duchenne muscular dystrophy or amyotrophic lateral sclerosis, has led to an intense search for new potential biomarkers. Biomarkers can be classified based on their clinical value into different categories: diagnostic biomarkers confirm the presence of a specific disease, prognostic biomarkers provide information about disease course, and therapeutic biomarkers are designed to predict or measure treatment response. Circulating biomarkers, as opposed to instrumental/invasive ones (e.g., muscle MRI or nerve ultrasound, muscle or nerve biopsy), are generally easier to access and less “time-consuming”. In addition to well-known creatine kinase, other promising molecules seem to be candidate biomarkers to improve the diagnosis, prognosis and prediction of therapeutic response, such as antibodies, neurofilaments, and microRNAs. However, there are some criticalities that can complicate their application: variability during the day, stability, and reliable performance metrics Citation: Barp, A.; Ferrero, A.; (e.g., accuracy, precision and reproducibility) across laboratories. In the present review, we discuss Casagrande, S.; Morini, R.; Zuccarino, the application of biochemical biomarkers (both validated and emerging) in the most common NMDs R. -

The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy
Review Neuromuscular Diseases Early Diagnosis and Treatment – The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy Eugenio Mercuri,1 Ros Quinlivan2 and Sylvie Tuffery-Giraud3 1. Catholic University, Rome, Italy; 2. Great Ormond Street Hospital and National Hospital for Neurology and Neurosurgery, London, UK; 3. Laboratory of Genetics of Rare Diseases (LGMR), University of Montpellier, Montpellier, France DOI: https://doi.org/10.17925/ENR.2018.13.1.31 he understanding of the natural history of Duchenne muscular dystrophy (DMD) is increasing rapidly and new treatments are emerging that have the potential to substantially improve the prognosis for patients with this disabling and life-shortening disease. For many, Thowever, there is a long delay between the appearance of symptoms and DMD diagnosis, which reduces the possibility of successful treatment. DMD results from mutations in the large dystrophin gene of which one-third are de novo mutations and two-thirds are inherited from a female carrier. Roughly 75% of mutations are large rearrangements and 25% are point mutations. Certain deletions and nonsense mutations can be treated whereas many other mutations cannot currently be treated. This emphasises the need for early genetic testing to identify the mutation, guide treatment and inform genetic counselling. Treatments for DMD include corticosteroids and more recently, ataluren has been approved in Europe, the first disease-modifying therapy for treating DMD caused by nonsense mutations. The use of ataluren in DMD is supported by positive results from phase IIb and phase III studies in which the treatment produced marked improvements in the 6-minute walk test, timed function tests such as the 10 m walk/run test and the 4-stair ascent/descent test compared with placebo. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Animal Models of Duchenne Muscular Dystrophy: from Basic Mechanisms to Gene Therapy Joe W
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 195-213 doi:10.1242/dmm.018424 REVIEW Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy Joe W. McGreevy1, Chady H. Hakim1, Mark A. McIntosh1 and Dongsheng Duan1,2,* ABSTRACT The identification of the disease-causing gene and the molecular Duchenne muscular dystrophy (DMD) is a progressive muscle- basis for the DMD and BMD phenotypes establishes the foundation wasting disorder. It is caused by loss-of-function mutations in the for DMD gene therapy (Fig. 2A). To mitigate muscle disease, one dystrophin gene. Currently, there is no cure. A highly promising can either restore the full-length transcript or express a truncated but therapeutic strategy is to replace or repair the defective dystrophin in-frame dystrophin gene (Duan, 2011; Goyenvalle et al., 2011; gene by gene therapy. Numerous animal models of DMD have been Konieczny et al., 2013; Mendell et al., 2012; Verhaart and Aartsma- developed over the last 30 years, ranging from invertebrate to large Rus, 2012). Several gene therapy strategies are currently under mammalian models. mdx mice are the most commonly employed development. They include replacing the mutated gene with a models in DMD research and have been used to lay the groundwork functional candidate gene (gene replacement) or repairing the for DMD gene therapy. After ~30 years of development, the field has defective gene by targeted correction and exon skipping (gene reached the stage at which the results in mdx mice can be validated repair). Currently, adeno-associated virus (AAV)-mediated gene and scaled-up in symptomatic large animals. -
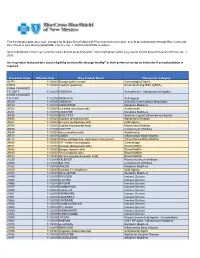
Procedure Code Effective Date Drug Product Name* Therapeutic
This list includes procedure code changes for Medical Benefit Specialty Pharmacy that may require benefit preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM) effective Jan. 1, 2020 for BCBSNM members. Items highlighted in blue may currently require benefit preauthorization. Items highlighted yellow may require benefit preauthorization effective Jan. 1, 2020. It is imperative that providers check eligibility and benefits through Availity® or their preferred vendor to determine if preauthorization is required. Procedure Code Effective Date Drug Product Name* Therapeutic Category 90378 1/1/2020 Synagis (palivizumab) Immunological Agent C9036 1/1/2020 Onpattro (patisiran) Small interfering RNA (siRNA) C9466 CHANGED TO J0517 1/1/2019 FASENRA Antiasthmatic - Monoclonal Antibodies C9493 CHANGED TO J1301 1/1/2019 RADICAVA ALS Agents J0129 1/1/2019 ORENCIA Selective Costimulation Modulators J0180 1/1/2019 FABRAZYME Metabolic Modifiers J0202 1/1/2020 Lemtrada (alemtuzumab) Autoimmune J0221 1/1/2019 LUMIZYME Metabolic Modifiers J0490 1/1/2019 BENLYSTA Systemic Lupus Erythematosus Agents J0565 1/1/2020 Zinplava (bezlotoxumab) Monoclonal Antibody J0567 1/1/2020 Brineura (cerliponase alfa) CNS Agents J0584 1/1/2020 Crysvita (burosumab-twza) Monoclonal Antibody J0598 1/1/2019 CINRYZE Complement Inhibitors J0638 1/1/2020 Ilaris (canakinumab) Autoimmune J0717 1/1/2019 CIMZIA Inflammatory Bowel Agents J0775 1/1/2020 Xiaflex (collagenase, clostridium histolyticum) Tissue Permeability Modifier J0800 1/1/2020 H.P. Acthar (corticotropin) -

NHS England Risdiplam EAMS Framework
FRAMEWORK OF ADVICE ON THE RISDIPLAM EARLY ACCESS TO MEDICINES SCHEME Background On 17 September 2020, Risdiplam was made available via the Early Access to Medicines Scheme (EAMS), details of which can be found at: https://www.gov.uk/government/publications/risdiplam-in-the-treatment-of-type-1- and-type-2-spinal-muscular-atrophy-sma-in-patients-2-months-of-age-and-older The EAMS scientific opinion issued to Roche Products Limited is for Risdiplam 0.75 mg/ml powder for oral solution in the treatment of type 1 and type 2 Spinal Muscular Atrophy (SMA) in patients 2 months and older who are not suitable for authorised treatments. The questions in the NHS England Blueteq form are attached for reference at Appendix A. The NHS England Nusinersen Clinical Panel, which is also established to offer advice more generally on the treatment and care of patients with spinal muscular atrophy, was asked to develop a framework of advice for clinicians to assist in interpreting the EAMS scientific opinion. The information below is a framework of advice for clinicians to assist in interpreting the EAMS scientific opinion and is not a directive from NHS England. The Clinical Panel understands that each patient case has unique factors and the treating physician must acknowledge that the patient fits the EAMS indication of not suitable for authorised treatments. This is in the context of nusinersen being available to eligible patients through a managed access agreement and onasemnogene abeparvovec being evaluated by NICE. The Clinical Panel is also able to offer advice on individual cases. -

Rxoutlook® 1St Quarter 2019
® RxOutlook 1st Quarter 2020 optum.com/optumrx a RxOutlook 1st Quarter 2020 Orphan drugs continue to feature prominently in the drug development pipeline In 1983 the Orphan Drug Act was signed into law. Thirty seven years later, what was initially envisioned as a minor category of drugs has become a major part of the drug development pipeline. The Orphan Drug Act was passed by the United States Congress in 1983 in order to spur drug development for rare conditions with high unmet need. The legislation provided financial incentives to manufacturers if they could demonstrate that the target population for their drug consisted of fewer than 200,000 persons in the United States, or that there was no reasonable expectation that commercial sales would be sufficient to recoup the developmental costs associated with the drug. These “Orphan Drug” approvals have become increasingly common over the last two decades. In 2000, two of the 27 (7%) new drugs approved by the FDA had Orphan Designation, whereas in 2019, 20 of the 48 new drugs (42%) approved by the FDA had Orphan Designation. Since the passage of the Orphan Drug Act, 37 years ago, additional regulations and FDA designations have been implemented in an attempt to further expedite drug development for certain serious and life threatening conditions. Drugs with a Fast Track designation can use Phase 2 clinical trials to support FDA approval. Drugs with Breakthrough Therapy designation can use alternative clinical trial designs instead of the traditional randomized, double-blind, placebo-controlled trial. Additionally, drugs may be approved via the Accelerated Approval pathway using surrogate endpoints in clinical trials rather than clinical outcomes. -

CP.PHAR.477 Risdiplam (Evrysdi)
Clinical Policy: Risdiplam (Evrysdi) Reference Number: CP.PHAR.477 Effective Date: 08.07.20 Last Review Date: 08.20 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Risdiplam (Evrysdi™) is a survival motor neuron 2 (SMN2) gene pre-mRNA splicing modifier. FDA Approved Indication(s) Evrysdi is indicated for the treatment of spinal muscular atrophy (SMA) in patients 2 months of age and older. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Evrysdi is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Spinal Muscular Atrophy (must meet all): 1. Diagnosis of SMA with documentation of both of the following (a and b): a. Genetic testing quantifying number of copies of SMN2 gene ≥ 1 but ≤ 4; b. Member is symptomatic; 2. Genetic testing confirms the presence of one of the following (a, b, or c): a. Homozygous deletions of SMN1 gene (e.g., absence of the SMN1 gene); b. Homozygous mutation in the SMN1 gene (e.g., biallelic mutations of exon 7); c. Compound heterozygous mutation in the SMN1 gene [e.g., deletion of SMN1 exon 7 (allele 1) and mutation of SMN1 (allele 2)]; 3. Prescribed by or in consultation with a neurologist; 4. Age ≥ 2 months; 5. Documentation of one of the following baseline scores (see Appendix D) (a or b): a. -

Innovationsreport 2020 Kurzfassung
Innovationsreport 2020 Auswertungsergebnisse von Routinedaten der Techniker Krankenkasse aus den Jahren 2017 bis 2018 Herausgeber: Gerd Glaeske Erstellt mit freundlicher Unterstützung der Techniker Krankenkasse (TK) 3 Herausgeber Prof. Dr. Gerd Glaeske Experten für ausgewählte Kapitel Prof. Dr. med. Janbernd Kirschner, Bonn Prof. Dr. med. Dieter Ukena, Bremen Prof. Dr. med. Barbara Schmalfeldt, Hamburg Prof. Dr. med. Wolfgang Schramm, München Autoren Prof. Dr. med. Karl Broich, Dr. Stanislava Dicheva‐Radev, Dörte Fuchs, Prof. Dr. Gerd Glaeske, Dr. Marion Haberkamp, Dr. Iris Hinneburg, Friederike Höfel, Prof. Dr. Janbernd Kirschner, Dr. Wiebke Löbker, Anja Lübs, Dr. André S. Morawetz, Lutz Muth, Dr. Frauke Naumann‐Winter, Linda Richter, Saskia Ritter, Dr. Kristin Sauer, Dr. Birgit Schindler unter Mitarbeit von Esra Aksoy, Friederike Höfel, Berit Marquardt, Linda Richter, Marle Wilhelm Anschrift: Universität Bremen, SOCIUM, Mary‐Somerville‐Str. 5, 28359 Bremen Aus Gründen der besseren Lesbarkeit wurde auf die Nennung beider geschlechtsspezifischer Formen verzichtet. Im Allgemeinen ist aber das jeweils andere Geschlecht ebenfalls gemeint. 2 Glossar .......................................................................................... 7 Vorwort zum Innovationsreport 2020 ...........................................15 Vorwort des Herausgebers ............................................................17 1 Einleitung ................................................................................19 2 Ziele und Methodik..................................................................33 -

06/01/2021– Unitedhealthcare Community Plan
UnitedHealthcare Community Plan of Kentucky Medical Policy Update Bulletin: June 2021 In This Issue Medical Policy Updates Page Updated • Pharmacogenetic Testing – Effective Jun. 1, 2021 ................................................................................................................................................................................................ 3 Revised • Articular Cartilage Defect Repairs – Effective Jul. 1, 2021 .................................................................................................................................................................................... 3 • Cell-Free Fetal DNA Testing – Effective Jul. 1, 2021 .............................................................................................................................................................................................. 6 • Implanted Electrical Stimulator for Spinal Cord – Effective Jul. 1, 2021 .............................................................................................................................................................. 8 • Lower Extremity Invasive Diagnostic and Endovascular Procedures – Effective Jul. 1, 2021 ............................................................................................................................ 9 Replaced/Retired • Femoroacetabular Impingement Syndrome – Effective Jun. 1, 2021 ................................................................................................................................................................