Prescription Drugs Requiring Prior Authorization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTE: the Following Are Shirley Ryan Abilitylab's Standard Charges As of Jan. 1, 2019. These Standard Charges Often Do Not
NOTE: The following are Shirley Ryan AbilityLab's standard charges as of Jan. 1, 2019. These standard charges often do not reflect what a patient or their insurance company/payer may be required to pay. Contact Patient Financial Services at 312-238-6039 for more information. Description Price 11-Deoxycortisol, Urine Random $531 12 Lead Ekg, Tracing Only $245 17 Hydroxycorticosteroids, Urine Timed $363 17 Hydroxyprogesterone, Serum $172 17 Ketosteroids, Urine Timed $273 17-Ketosteroids with Creatinine, Urine Random $273 2D Gait Analysis Charge(95999) $2,174 5' Nucleotidase, Serum $269 5.5 PDC PEDS CUFFED TRACH $226 5-HIAA-24 Hr Urine $99 A4566 Shoulder sling or vest design, abduction res $226 A4595 FES Electrode, each $38 A6545 Gradient Compression Wrap, Non-Elastic, Belo $171 ABO Rh Type $106 Above Knee Nylon Hose PR $83 Above Knee Suction Sheath $131 Above knee, for proximal femoral focal deficiency, $14,344 Above knee, molded socket, open end, SACH foot, en $10,751 Above knee, molded socket, single axis constant fr $10,935 Above knee, short prosthesis, no knee joint (""stu_L5210 $8,682 Above knee, short prosthesis, no knee joint (""stu_L5220 $7,429 ACAPELLA DH GREEN VIBRATORY PEP W/MOUTHPIECE DEVIC $181 Acetabuloplasty (27120) $4,493 Acetone, Serum $27 Acetylcholine Receptor Binding Antibody $405 Acid Phosphatase $66 Acid Phosphatase, Prostatic fraction $66 ACNE SURGERY MILIA CYSTS (10040) $530 Acorn Nebulizer $174 Activated PTT/Partial Thromboplastin Time $109 Active Wound Care > 20 cm Units $171 Active Wound Care/20 cm or < Units $171 Acupuncture with E-Stim. Each additional 15 minute $65 Acupuncture with E-Stim. -

Dexamethasone Sodium Phosphate Injection, USP)
HEXADROL® PHOSPHATE INJECTION (dexamethasone sodium phosphate injection, USP) DESCRIPTION — Hexadrol® Phosphate Injection (dexamethasone sodium phosphate injection, USP) is a water-soluble inorganic ester of dexamethasone which produces a rapid response even when injected intramuscularly. Dexamethasone Sodium Phosphate, C22H28FNa2O8P, has a molecular weight of 516.41 and chemically is Pregn-4-ene-3, 20-dione, 9-fluoro-11, 17-dihydroxy-16-methyl-21 (phosphonooxy)-, disodium salt, (11β, 16α). It occurs as a white to creamy white powder, is exceedingly hygroscopic, is soluble in water and its solutions have a pH between 7.5 and 10.5. It has the following structural formula: Hexadrol® Phosphate Injection is available in 4 mg/mL and 10 mg/mL concentrations. Each mL of Hexadrol® Phosphate Injection 4 mg/mL, contains dexamethasone sodium phosphate, USP equivalent to 4 mg dexamethasone phosphate; 1 mg sodium sulfite; 10 mg benzyl alcohol (preservative). Made isotonic with sodium citrate. pH adjusted with citric acid or sodium hydroxide. 1 Reference ID: 3593917 Each mL of Hexadrol® Phosphate Injection 10 mg/mL, contains dexamethasone sodium phosphate, USP equivalent to 10 mg dexamethasone phosphate; 1.5 mg sodium sulfite; 10 mg benzyl alcohol (preservative). Made isotonic with sodium citrate. pH adjusted with citric acid or sodium hydroxide. ACTIONS — Naturally occurring glucocorticoids (hydrocortisone), which also have salt- retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems. Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli. -

Vertebroplasty (Repair of Vertebrae with Bone Cement)
Patient information – Radiology Unit Tel 0118 322 7991 Vertebroplasty (repair of vertebrae with bone cement) Introduction This leaflet tells you about the procedure known as “vertebroplasty”, explains what is involved, the benefits and possible risks. It is not meant to replace informed discussion between you and your doctor but can act as a starting point for such a discussion. You should have plenty of time to discuss this procedure with the doctor who will be performing the procedure. What is vertebroplasty? Vertebroplasty is a procedure used to strengthen a collapsed spinal vertebra that has fractured and usually lost height due to osteoporosis or, less commonly, tumour infiltration or trauma. Vertebroplasty is primarily aimed at relieving the patient’s pain, to allow a rapid return to the previous level of activity, and secondarily to prevent further vertebral collapse. It is minimally invasive which means that patients suffer less disruption, face less risk and recover more quickly than with conventional open surgery. It is most effective when the fracture is relatively recent and particularly in patients who are very debilitated by the pain of the fracture or who cannot tolerate adequate quantities of painkilling medication. In some circumstances, vertebroplasty can be very effective even if the fracture is many months or even years old. Vertebroplasty is accomplished by injecting orthopaedic cement through a needle into the fractured bone; essentially forming an internal cast to stabilise the fracture similar to the principle of using plaster cast on an arm fracture. Typically, vertebroplasty is recommended if simpler treatments, such as bed rest, back bracing or pain medication have been ineffective, or if the side effects of analgesia have become problematic, such as causing stomach ulcers or drowsiness. -

Oxford® Policy Update Bulletin: December 2015 Oxford
Oxford December 2015 policy update bulletin Medical & Administrative Policy Updates UnitedHealthcare respects the expertise of the physicians, health care professionals, and their staff who participate in our network. Our goal is to support you and your patients in making the most informed decisions regarding the choice of quality and cost-effective care, and to support practice staff with a simple and predictable administrative experience. The Policy Update Bulletin was developed to share important information regarding Oxford® Medical and Administrative Policy updates.* *Where information in this bulletin conflicts with applicable state and/or federal law, Oxford® follows such applicable federal and/or state law Oxford Oxford® Medical and Administrative Policy Updates Overview This bulletin provides complete details on Oxford® Medical and Policy Update Classifications Administrative Policy updates. The appearance of a service or New procedure in this bulletin indicates only that Oxford® has recently New clinical coverage criteria and/or documentation review requirements adopted a new policy and/or updated, revised, replaced or have been adopted for a service, procedure, test, or device retired an existing policy; it does not imply that Oxford® provides Updated coverage for the service or procedure. In the event of an An existing policy has been reviewed and changes have not been made inconsistency or conflict between the information provided in this to the clinical coverage criteria or documentation review requirements; bulletin and the posted policy, the provisions of the posted policy however, items such as the clinical evidence, FDA information, and/or will prevail. Note that most benefit plan documents exclude from list(s) of applicable codes may have been updated benefit coverage health services identified as investigational or unproven/not medically necessary. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Joint Injection As an Out-Patient Procedure
This leaflet is available in large print, Braille and on tape. Please contact Geoff Pennock on 0151 604 7289. Joint Injection As An Out-Patient Procedure Wirral University Teaching Hospital NHS Foundation Trust operates a No Smoking Policy. Please refrain from smoking on site. Information for Patients Orthopaedic Clinic Author: K Howard Date of Publication: June 2013 Date for Review: June 2015 Reference: Taken from the BUPA information leaflet. Having an injection into the joint. PL/00293/Surgical-Ortho/0613 Do Not Copy. For further copies contact Supplies quoting the PL number. This leaflet provides some information The risks of a joint injection In order to get maximum benefit from about joint injections. Please take Joint injections are a commonly your injection;- this home for further referral. Your performed and are generally safe consultant has prescribed this treatment procedures. Adverse reactions and • Rest the joint for a day. You may to meet your individual needs. Please side effects are rare; some patients need to take time off work, this will raise any concerns or questions you experience quite a painful reaction depend on the type of job you do – have and we will endeavour to answer after the injection lasting two-four your consultant will advise. them. days, this will normally settle. However, • You may need to take occasional if you do experience excessive pain, pain relieving medication e.g. The benefits of a joint injection swelling or you become unwell with Paracetamol, or anti- inflammatory The purpose of administering a a temperature, contact your doctor or tablets. joint injection is to reduce pain, the clinic immediately. -

The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy
Review Neuromuscular Diseases Early Diagnosis and Treatment – The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy Eugenio Mercuri,1 Ros Quinlivan2 and Sylvie Tuffery-Giraud3 1. Catholic University, Rome, Italy; 2. Great Ormond Street Hospital and National Hospital for Neurology and Neurosurgery, London, UK; 3. Laboratory of Genetics of Rare Diseases (LGMR), University of Montpellier, Montpellier, France DOI: https://doi.org/10.17925/ENR.2018.13.1.31 he understanding of the natural history of Duchenne muscular dystrophy (DMD) is increasing rapidly and new treatments are emerging that have the potential to substantially improve the prognosis for patients with this disabling and life-shortening disease. For many, Thowever, there is a long delay between the appearance of symptoms and DMD diagnosis, which reduces the possibility of successful treatment. DMD results from mutations in the large dystrophin gene of which one-third are de novo mutations and two-thirds are inherited from a female carrier. Roughly 75% of mutations are large rearrangements and 25% are point mutations. Certain deletions and nonsense mutations can be treated whereas many other mutations cannot currently be treated. This emphasises the need for early genetic testing to identify the mutation, guide treatment and inform genetic counselling. Treatments for DMD include corticosteroids and more recently, ataluren has been approved in Europe, the first disease-modifying therapy for treating DMD caused by nonsense mutations. The use of ataluren in DMD is supported by positive results from phase IIb and phase III studies in which the treatment produced marked improvements in the 6-minute walk test, timed function tests such as the 10 m walk/run test and the 4-stair ascent/descent test compared with placebo. -

CLINICAL GUIDELINES CMM-200: Epidural Steroid Injections (ESI) Version 1.0 Effective February 14, 2020
CLINICAL GUIDELINES CMM-200: Epidural Steroid Injections (ESI) Version 1.0 Effective February 14, 2020 Clinical guidelines for medical necessity review of Comprehensive Musculoskeletal Management Services. © 2019 eviCore healthcare. All rights reserved. Comprehensive Musculoskeletal Management Guidelines V1.0 CMM-200: Epidural Steroid Injections(ESI) CMM-200.1: Definitions 3 CMM-200.2: General Guidelines 4 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 5 CMM-200.4: Indications: Epidural Steroid Injections (Transforaminal, Interlaminar, or Caudal) 5 CMM-200.5: Non-Indications: SNRB 6 CMM-200.6: Non-Indications: ESI 7 CMM-200.7: Procedure (CPT®) Codes 8 CMM-200.8: References 10 ______________________________________________________________________________________________________ ©2020 eviCore healthcare. All Rights Reserved. Page 2 of 16 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Comprehensive Musculoskeletal Management Guidelines V1.0 CMM -200.1: Definitions Transforaminal epidural steroid injection (TFESI) is a therapeutic injection of contrast (absent allergy to contrast) performed at a single or multiple spinal levels, followed by the introduction of a corticosteroid and possibly a local anesthetic by inserting a needle into the neuroforamen under fluoroscopic or computed tomography (CT) guidance. Selective Nerve Root Block (SNRB) is a diagnostic injection of contrast (absent allergy to contrast) of a single nerve root to assist with surgical planning, followed by the introduction of a local anesthetic by inserting a needle into the neuroforamen under fluoroscopic or computed tomography (CT) guidance. SNRBs are erroneously referred to as transforaminal epidural steroid injection (TFESI), although technically SNRBs involve the introduction of anesthetic only and are used for diagnostic purposes. Selective nerve root blocks (SNRBs) performed for the purpose of treating pain (i.e., repeat SNRB at the same level) may be termed therapeutic selective nerve root blocks. -

Sacroiliac Joint Injection
Dr Sean White Consultant in Pain Medicine Sacroiliac joint Injection 1 Introduction Before you agree to have your sacroiliac injections, it is sensible to know all you can about it. This means knowing why you may need sacroiliac injections, what the procedure is, and what it will be like, if there are any risks, and if there are any alternatives. Even if you are not keen on the details, getting an overall picture is helpful. This leaflet is a good starting point. It does not cover everything, so do mention any particular worries you may have. Ask for more information at any time. Patients, their pain problem and treatments all vary to a degree. But the leaflet should help you make the best decision. You will need to sign a consent form. This will happen just before the procedure at the hospital. This consent form records what you have agreed to. It is flexible enough to cover the unexpected. Please make sure everything is quite clear to you. Mention anything you do not wish to have done. You can change your mind even after signing the consent form. What are sacroiliac joint injections? Sacroiliac joints are the large pair of joints that hold the pelvis onto the spinal column. They help keep the spine straight and firm to hold your body weight. At the same time they allow limited movement so that you can bend, stretch and rotate. With injury or simple ‘wear and tear’ these joints can become painful. Injections into these joints are used to treat pain in that area. -

Animal Models of Duchenne Muscular Dystrophy: from Basic Mechanisms to Gene Therapy Joe W
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 195-213 doi:10.1242/dmm.018424 REVIEW Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy Joe W. McGreevy1, Chady H. Hakim1, Mark A. McIntosh1 and Dongsheng Duan1,2,* ABSTRACT The identification of the disease-causing gene and the molecular Duchenne muscular dystrophy (DMD) is a progressive muscle- basis for the DMD and BMD phenotypes establishes the foundation wasting disorder. It is caused by loss-of-function mutations in the for DMD gene therapy (Fig. 2A). To mitigate muscle disease, one dystrophin gene. Currently, there is no cure. A highly promising can either restore the full-length transcript or express a truncated but therapeutic strategy is to replace or repair the defective dystrophin in-frame dystrophin gene (Duan, 2011; Goyenvalle et al., 2011; gene by gene therapy. Numerous animal models of DMD have been Konieczny et al., 2013; Mendell et al., 2012; Verhaart and Aartsma- developed over the last 30 years, ranging from invertebrate to large Rus, 2012). Several gene therapy strategies are currently under mammalian models. mdx mice are the most commonly employed development. They include replacing the mutated gene with a models in DMD research and have been used to lay the groundwork functional candidate gene (gene replacement) or repairing the for DMD gene therapy. After ~30 years of development, the field has defective gene by targeted correction and exon skipping (gene reached the stage at which the results in mdx mice can be validated repair). Currently, adeno-associated virus (AAV)-mediated gene and scaled-up in symptomatic large animals. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -
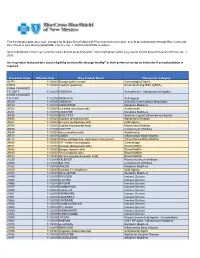
Procedure Code Effective Date Drug Product Name* Therapeutic
This list includes procedure code changes for Medical Benefit Specialty Pharmacy that may require benefit preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM) effective Jan. 1, 2020 for BCBSNM members. Items highlighted in blue may currently require benefit preauthorization. Items highlighted yellow may require benefit preauthorization effective Jan. 1, 2020. It is imperative that providers check eligibility and benefits through Availity® or their preferred vendor to determine if preauthorization is required. Procedure Code Effective Date Drug Product Name* Therapeutic Category 90378 1/1/2020 Synagis (palivizumab) Immunological Agent C9036 1/1/2020 Onpattro (patisiran) Small interfering RNA (siRNA) C9466 CHANGED TO J0517 1/1/2019 FASENRA Antiasthmatic - Monoclonal Antibodies C9493 CHANGED TO J1301 1/1/2019 RADICAVA ALS Agents J0129 1/1/2019 ORENCIA Selective Costimulation Modulators J0180 1/1/2019 FABRAZYME Metabolic Modifiers J0202 1/1/2020 Lemtrada (alemtuzumab) Autoimmune J0221 1/1/2019 LUMIZYME Metabolic Modifiers J0490 1/1/2019 BENLYSTA Systemic Lupus Erythematosus Agents J0565 1/1/2020 Zinplava (bezlotoxumab) Monoclonal Antibody J0567 1/1/2020 Brineura (cerliponase alfa) CNS Agents J0584 1/1/2020 Crysvita (burosumab-twza) Monoclonal Antibody J0598 1/1/2019 CINRYZE Complement Inhibitors J0638 1/1/2020 Ilaris (canakinumab) Autoimmune J0717 1/1/2019 CIMZIA Inflammatory Bowel Agents J0775 1/1/2020 Xiaflex (collagenase, clostridium histolyticum) Tissue Permeability Modifier J0800 1/1/2020 H.P. Acthar (corticotropin)