Med Pharm PA List to Build Pdfs.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Aetna Pharmacy Drug Guide Aetna Premier Plus Plan
Plan for your best health 2019 Aetna Pharmacy Drug Guide Aetna Premier Plus Plan aetna.com 05.02.414.1 O (12/19) Aetna is the brand name used for products and services provided by one or more of the Aetna group of subsidiary companies, including Aetna Life Insurance Company and its affiliates (Aetna). Aetna Pharmacy Management refers to an internal business unit of Aetna Health Management, LLC. Aetna Pharmacy Management administers, but does not offer, insure or otherwise underwrite the prescription drug benefits portion of your health plan and has no financial responsibility therefor. 2019 Aetna Commercial Plan (Premier Plus) Table of Contents INFORMATIONAL SECTION..................................................................................................................7 *5-HT4 RECEPTOR AGONISTS*** - DRUGS FOR THE STOMACH................................................ 18 *ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS* - DRUGS FOR THE NERVOUS SYSTEM.................................................................................................................................18 *AGENTS FOR NARCOTIC WITHDRAWAL*** - DRUGS FOR ADDICTION............................... 22 *AGENTS FOR OPIOID WITHDRAWAL*** - DRUGS FOR ADDICTION......................................22 *AMEBICIDES* - DRUGS FOR INFECTIONS.....................................................................................22 *AMINO ACIDS*** - DRUGS FOR NUTRITION................................................................................ 22 *AMINOGLYCOSIDES* - DRUGS FOR -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Prescription Drugs Requiring Prior Authorization
PRESCRIPTION DRUGS REQUIRING PRIOR AUTHORIZATION Revised 10/16 As part of our drug utilization management program, members must request and receive prior authorization for certain prescription drugs in order to use their prescription drug benefits. Below is a list of drugs that currently require prior authorization. This list will be updated periodically as new drugs that require prior authorization are introduced. As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. The Schedule of Benefits contains a list of drug categories that require prior authorization. Prior authorization requests are processed by our pharmacy benefit manager, Express Scripts, Inc. (ESI). Physicians must call ESI to obtain an authorization. (1-800-842-2015). Drug Name Generic Name Drug Classification Abstral fentanyl citrate oral tablet Controlled Dangerous Substance Accu-Chek Test Strips blood glucose test strips Blood Glucose Test Strips Actemra tocilizumab Monoclonal Antibody Acthar corticotropin Hormone Actimmune interferon gamma 1b Interferon Actiq fentanyl citrate OTFC Controlled Dangerous Substance Adcirca tadalafil Pulmonary Vasodilator Adempas riociguat Pulmonary Vasodilator Adlyxin lixisenatide Type 2 Diabetes Advocate Test Strips blood glucose test strips Blood Glucose Test Strips Aerospan** flunisolide Corticosteroids (Inhaled) Afrezza insulin Insulin (inhaled) Ampyra dalfampridine Multiple Sclerosis Agent Altoprev** lovastatin Cholesterol Alvesco** ciclesonide Corticosteroids -

The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy
Review Neuromuscular Diseases Early Diagnosis and Treatment – The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy Eugenio Mercuri,1 Ros Quinlivan2 and Sylvie Tuffery-Giraud3 1. Catholic University, Rome, Italy; 2. Great Ormond Street Hospital and National Hospital for Neurology and Neurosurgery, London, UK; 3. Laboratory of Genetics of Rare Diseases (LGMR), University of Montpellier, Montpellier, France DOI: https://doi.org/10.17925/ENR.2018.13.1.31 he understanding of the natural history of Duchenne muscular dystrophy (DMD) is increasing rapidly and new treatments are emerging that have the potential to substantially improve the prognosis for patients with this disabling and life-shortening disease. For many, Thowever, there is a long delay between the appearance of symptoms and DMD diagnosis, which reduces the possibility of successful treatment. DMD results from mutations in the large dystrophin gene of which one-third are de novo mutations and two-thirds are inherited from a female carrier. Roughly 75% of mutations are large rearrangements and 25% are point mutations. Certain deletions and nonsense mutations can be treated whereas many other mutations cannot currently be treated. This emphasises the need for early genetic testing to identify the mutation, guide treatment and inform genetic counselling. Treatments for DMD include corticosteroids and more recently, ataluren has been approved in Europe, the first disease-modifying therapy for treating DMD caused by nonsense mutations. The use of ataluren in DMD is supported by positive results from phase IIb and phase III studies in which the treatment produced marked improvements in the 6-minute walk test, timed function tests such as the 10 m walk/run test and the 4-stair ascent/descent test compared with placebo. -

Spotlight on Market Access Actionable Understandings from AIS Health’S In-Depth Coverage
Spotlight on Market Access Actionable understandings from AIS Health’s in-depth coverage September 16, 2019 Recent Situations Stress That Data Is More Important Than Ever to FDA, Drug Uptake 2 Clinical Trials by Indication: Q2 2019 A pair of drugmakers and the FDA found themselves in the news lately, but it’s safe to say it wasn’t for the reasons they would prefer. Both situations Reality Check: PCSK9 Inhibitors 8 stress the importance of data needed to secure product approvals, and, per- haps, payer and provider uptake. On Aug. 6, the FDA put out a statement addressing “data accuracy issues” with Zolgensma (onasemnogene abeparvovec-xioi), a new gene ther- apy to treat spinal muscular atrophy in people less than 2 years old who have bi-allelic mutations in the survival motor neuron 1 gene, including those who are presymptomatic when diagnosed. The one-time therapy has the distinction of being the most expensive drug in the world, with a price tag of $2.1 million. The FDA approved the drug from AveXis, Inc. on May 24 (SMA 7/1/19, p. 6). On June 28, AveXis — which was acquired by Novartis AG last year — notified the agency that there was “a data manipulation issue that impacts the accuracy of certain data from product testing performed in animals sub- mitted in the biologics license application (BLA) and reviewed by the FDA.” continued on p. 3 Report Reveals That Almost 100 RM/AT Products Are in Phase III Clinical Trials This past quarter saw two new gene therapies: Novartis AG subsidiary AveXis, Inc.’s Zolgensma (onasemnogene abeparvovec-xioi) received FDA approval May 24 for the treatment of spinal muscular atrophy (SMA 7/1/19, p. -

Ophthalmologic Policy: Vascular Endothelial Growth Factor (VEGF) Inhibitors
UnitedHealthcare® Community Plan Medical Benefit Drug Policy Ophthalmologic Policy: Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: CS2021D0042S Effective Date: March 1, 2021 Instructions for Use Table of Contents Page Related Community Plan Policies Application.......................................................................................... 1 • Macular Degeneration Treatment Procedures Coverage Rationale ........................................................................... 1 • Maximum Dosage and Frequency Definitions ........................................................................................... 3 • Oncology Medication Clinical Coverage Applicable Codes .............................................................................. 3 Background ........................................................................................ 3 Commercial Policy Clinical Evidence .............................................................................23 • Ophthalmologic Policy: Vascular Endothelial Growth U.S. Food and Drug Administration ..............................................34 Factor (VEGF) Inhibitors Centers for Medicare and Medicaid Services .............................35 References .......................................................................................35 Policy History/Revision Information..............................................39 Instructions for Use .........................................................................39 Application This Medical Benefit -

Animal Models of Duchenne Muscular Dystrophy: from Basic Mechanisms to Gene Therapy Joe W
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 195-213 doi:10.1242/dmm.018424 REVIEW Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy Joe W. McGreevy1, Chady H. Hakim1, Mark A. McIntosh1 and Dongsheng Duan1,2,* ABSTRACT The identification of the disease-causing gene and the molecular Duchenne muscular dystrophy (DMD) is a progressive muscle- basis for the DMD and BMD phenotypes establishes the foundation wasting disorder. It is caused by loss-of-function mutations in the for DMD gene therapy (Fig. 2A). To mitigate muscle disease, one dystrophin gene. Currently, there is no cure. A highly promising can either restore the full-length transcript or express a truncated but therapeutic strategy is to replace or repair the defective dystrophin in-frame dystrophin gene (Duan, 2011; Goyenvalle et al., 2011; gene by gene therapy. Numerous animal models of DMD have been Konieczny et al., 2013; Mendell et al., 2012; Verhaart and Aartsma- developed over the last 30 years, ranging from invertebrate to large Rus, 2012). Several gene therapy strategies are currently under mammalian models. mdx mice are the most commonly employed development. They include replacing the mutated gene with a models in DMD research and have been used to lay the groundwork functional candidate gene (gene replacement) or repairing the for DMD gene therapy. After ~30 years of development, the field has defective gene by targeted correction and exon skipping (gene reached the stage at which the results in mdx mice can be validated repair). Currently, adeno-associated virus (AAV)-mediated gene and scaled-up in symptomatic large animals. -
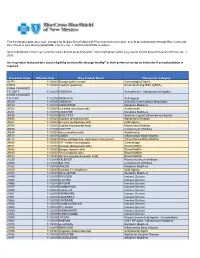
Procedure Code Effective Date Drug Product Name* Therapeutic
This list includes procedure code changes for Medical Benefit Specialty Pharmacy that may require benefit preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM) effective Jan. 1, 2020 for BCBSNM members. Items highlighted in blue may currently require benefit preauthorization. Items highlighted yellow may require benefit preauthorization effective Jan. 1, 2020. It is imperative that providers check eligibility and benefits through Availity® or their preferred vendor to determine if preauthorization is required. Procedure Code Effective Date Drug Product Name* Therapeutic Category 90378 1/1/2020 Synagis (palivizumab) Immunological Agent C9036 1/1/2020 Onpattro (patisiran) Small interfering RNA (siRNA) C9466 CHANGED TO J0517 1/1/2019 FASENRA Antiasthmatic - Monoclonal Antibodies C9493 CHANGED TO J1301 1/1/2019 RADICAVA ALS Agents J0129 1/1/2019 ORENCIA Selective Costimulation Modulators J0180 1/1/2019 FABRAZYME Metabolic Modifiers J0202 1/1/2020 Lemtrada (alemtuzumab) Autoimmune J0221 1/1/2019 LUMIZYME Metabolic Modifiers J0490 1/1/2019 BENLYSTA Systemic Lupus Erythematosus Agents J0565 1/1/2020 Zinplava (bezlotoxumab) Monoclonal Antibody J0567 1/1/2020 Brineura (cerliponase alfa) CNS Agents J0584 1/1/2020 Crysvita (burosumab-twza) Monoclonal Antibody J0598 1/1/2019 CINRYZE Complement Inhibitors J0638 1/1/2020 Ilaris (canakinumab) Autoimmune J0717 1/1/2019 CIMZIA Inflammatory Bowel Agents J0775 1/1/2020 Xiaflex (collagenase, clostridium histolyticum) Tissue Permeability Modifier J0800 1/1/2020 H.P. Acthar (corticotropin) -

Imaging Tumor Angiogenesis
Imaging tumor angiogenesis Kristy Red-Horse, Napoleone Ferrara J Clin Invest. 2006;116(10):2585-2587. https://doi.org/10.1172/JCI30058. Commentary Since the discovery of vascular-specific growth factors with angiogenic activity, there has been a significant effort to develop cancer drugs that restrict tumorigenesis by targeting the blood supply. In this issue of the JCI, Mancuso et al. use mouse models to better understand the plasticity of the tumor vasculature in the face of antiangiogenic therapy (see the related article beginning on page 2610). They describe a rapid regrowth of the tumor vasculature following withdrawal of VEGFR inhibitors, emphasizing the importance of fully understanding the function of these and similar treatments used in the clinic at the cellular and molecular level. Find the latest version: https://jci.me/30058/pdf commentaries Imaging tumor angiogenesis Kristy Red-Horse and Napoleone Ferrara Genentech Inc., South San Francisco, California, USA. Since the discovery of vascular-specific growth factors with angiogenic activ- in 1 week (Figure 1). In accordance with ity, there has been a significant effort to develop cancer drugs that restrict previous findings (4, 6), posttreatment tumorigenesis by targeting the blood supply. In this issue of the JCI, Mancuso angiogenic growth correlated with the et al. use mouse models to better understand the plasticity of the tumor vas- disappearance of empty basement mem- culature in the face of antiangiogenic therapy (see the related article begin- brane sleeves, suggesting that regrowth ning on page 2610). They describe a rapid regrowth of the tumor vasculature occurred along these structures (Figure 1). -

Curriculum Vitae
Curriculum Vitae PERSONAL INFORMATION Ulrike Schara WORK EXPERIENCE 1991- 1997 Education in Paediatrics Paediatric Outpatient Centre,City Hospital Gelsenkirchen. (Germany) Paediatric Outpatient Centre. City Hospital Gelsenkirchen.<br/>Childrens Hospital, Ruhr University Bochum<br/> 1998- 2002 Senior Neuropediatrics Children's University Hospital Bochum (Germany) . 2002- 2003 Head of Neuropediatrics Children's University Hospital Bochum (Germany) 2004- 2006 Head of Neuropediatrics Children's Hospital, City of Neuss (Germany) 2007- Present Head of the Neuropaediatric Outpatient Centre and Consultant for Neuropaediatrics University of Essen (Germany) EDUCATION AND TRAINING 1991- 2012 MD, Professor Ruhr University of Bochum, Germany University of Essen (Germany) ADDITIONAL INFORMATION Expertise Publications Publcations of the past 5 years 1.Rudnik-Schöneborn S, Tölle D, Senderek J, Eggermann K, Elbracht M, Kornak U, von der Hagen M, Kirschner J, Leube B, Müller-Felber W, Schara U, von Au K, Wieczorek D, Bußmann C, Zerres K. Diagnostic algorithms in Charcot-Marie-Tooth neuropathies: experiences from a German genetic laboratory on the basis of 1206 index patients. Clin Genet. 2016 Jan;89(1):34-43 2.Byrne S, Jansen L, U-King-Im JM, Siddiqui A, Lidov HG, Bodi I, Smith L, Mein R, Cullup T, Dionisi-Vici C, Al-Gazali L, Al-Owain M, Bruwer Z, Al Thihli K, El-Garhy R, Flanigan KM, Manickam K, Zmuda E, Banks W, Gershoni-Baruch R, Mandel H, Dagan E, Raas-Rothschild A, Barash H, Filloux F, Creel D, Harris M, Hamosh A, Kölker S, Ebrahimi-Fakhari D, Hoffmann GF, Manchester D, Boyer PJ, Manzur AY, Lourenco CM, Pilz DT, Kamath A, Prabhakar P, Rao VK, Rogers RC, Ryan MM, Brown NJ, McLean CA, Said E, Schara U, Stein A, Sewry C, Travan L, Wijburg FA, Zenker M, Mohammed S, Fanto M, Gautel M, Jungbluth H. -

Immunoprophylaxis of Influenza Using AAV Vector Delivery of Cross
Immunoprophylaxis of Influenza using AAV Vector Delivery of Cross-Subtype Neutralizing Single Domain Antibodies JOANNE MARIE M. DEL ROSARIO Thesis submitted for the degree of Doctor of Philosophy Infection and Immunity University College London 2020 To Chris, as fate would have it. To Teki, thank you for everything. 2 DECLARATION I, Joanne Marie M. Del Rosario, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. __________________________ 3 ABSTRACT Cross-subtype neutralizing single domain antibodies against influenza present new opportunities for immunoprophylaxis and pandemic preparedness. Their simple modular structure and single open reading frame format are highly amenable to gene therapy-mediated delivery. R1a-B6, an alpaca-derived single domain antibody (nanobody), that is capable of potent cross-subtype neutralization in vitro of H1N1, H5N1, H2N2, and H9N2 influenza viruses, through binding to a highly conserved epitope in the influenza hemagglutinin stem region, was previously described. To evaluate the potential of R1a-B6 for immunoprophylaxis via adeno-associated viral (AAV) vector delivery, it was reformatted as Fc fusions of mouse IgG1 (ADCC-) and IgG2a (ADCC+) isotypes. This is also to extend R1a-B6’s half-life and to assess the requirement for ADCC for efficacy of R1a-B6 in vitro and in vivo. It was found that reformatted R1a-B6 of either mouse IgG isotype retained its potent binding and neutralization activity against different Group I influenza A subtypes in vitro. The findings in this study also demonstrate that a single intramuscular injection in mice of AAV encoding R1a-B6-Fc was able to drive sustained high-level expression (0.5–1.1 mg/mL) of the nanobody-Fc in sera with no evidence of reduction for up to 6 months. -

020 Medicare Advantage Part B Step Therapy
Medicare Medical Policy Medicare Advantage Part B Step Therapy Medicare HMO BlueSM and Medicare PPO BlueSM Members Policy Number: 020 Related Policies • Quality Care Cancer Program (Medical Oncology), #099 • Supportive Care Treatments for Patients with Cancer, #105 This policy will only manage non-oncology indications for drugs with both oncology and non- oncology indications. For the management of oncology or supportive care indications, please see related policies above that are managed by AIM (Medical Policy #099 and #105). Note: All preservice authorization requests may be submitted to BCBSMA Clinical Pharmacy Operations by completing the preservice authorization form on the last page of this document. Prescribers may also call BCBSMA Pharmacy Operations department at (800) 366-7778 to request a preservice authorization verbally. Table of Contents NCD/LCD/Article ........................................................................................................................................... 2 Drug Class: Granulocyte Colony Stimulants (filgrastim): Zarxio; Neupogen, Nivestym ............... 2 Drug Class: Erythropoiesis Stimulants: Retacrit; Aranesp, Epogen, Mircera, Procrit ................. 2 Device Class: Hyaluronate and Derivatives: Hyalgan, Hymovis, Synvisc; Durolane, Euflexxa, Gel-One, Gelsyn-3, Genvisc, Monovisc, Orthovisc, Supartz Fx, Triluron, Trivisc, Visco-3 ... 2 Drug Class: Tumor Necrosis Factor (TNF) Blocking Agents: Inflectra; Remicade, Renflexis, Avsola ..........................................................................................................................................................