Eteplirsen NDA 206488
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Opportunities and Challenges for Antisense Oligonucleotide Therapies
Received: 10 January 2020 Revised: 23 April 2020 Accepted: 8 May 2020 DOI: 10.1002/jimd.12251 REVIEW ARTICLE Opportunities and challenges for antisense oligonucleotide therapies Elsa C. Kuijper1 | Atze J. Bergsma2,3 | W.W.M. Pim Pijnappel2,3 | Annemieke Aartsma-Rus1 1Department of Human Genetics, Leiden University Medical Center, Leiden, The Abstract Netherlands Antisense oligonucleotide (AON) therapies involve short strands of modi- 2Department of Pediatrics, Center for fied nucleotides that target RNA in a sequence-specific manner, inducing Lysosomal and Metabolic Diseases, targeted protein knockdown or restoration. Currently, 10 AON therapies Erasmus Medical Center, Rotterdam, The Netherlands have been approved in the United States and Europe. Nucleotides are chem- 3Department of Clinical Genetics, Center ically modified to protect AONs from degradation, enhance bioavailability for Lysosomal and Metabolic Diseases, and increase RNA affinity. Whereas single stranded AONs can efficiently Erasmus Medical Center, Rotterdam, The Netherlands be delivered systemically, delivery of double stranded AONs requires capsulation in lipid nanoparticles or binding to a conjugate as the uptake Correspondence enhancing backbone is hidden in this conformation. With improved chem- Annemieke Aartsma-Rus, LUMC Postzone S4-P, Albinusdreef 2, 2333 ZA istry, delivery vehicles and conjugates, doses can be lowered, thereby reduc- Leiden, The Netherlands. ing the risk and occurrence of side effects. AONs can be used to knockdown Email: [email protected] or restore levels of protein. Knockdown can be achieved by single stranded Communicating Editor: Carla E. Hollak or double stranded AONs binding the RNA transcript and activating RNaseH-mediated and RISC-mediated degradation respectively. Transcript binding by AONs can also prevent translation, hence reducing protein levels. -

Prescription Drugs Requiring Prior Authorization
PRESCRIPTION DRUGS REQUIRING PRIOR AUTHORIZATION Revised 10/16 As part of our drug utilization management program, members must request and receive prior authorization for certain prescription drugs in order to use their prescription drug benefits. Below is a list of drugs that currently require prior authorization. This list will be updated periodically as new drugs that require prior authorization are introduced. As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. The Schedule of Benefits contains a list of drug categories that require prior authorization. Prior authorization requests are processed by our pharmacy benefit manager, Express Scripts, Inc. (ESI). Physicians must call ESI to obtain an authorization. (1-800-842-2015). Drug Name Generic Name Drug Classification Abstral fentanyl citrate oral tablet Controlled Dangerous Substance Accu-Chek Test Strips blood glucose test strips Blood Glucose Test Strips Actemra tocilizumab Monoclonal Antibody Acthar corticotropin Hormone Actimmune interferon gamma 1b Interferon Actiq fentanyl citrate OTFC Controlled Dangerous Substance Adcirca tadalafil Pulmonary Vasodilator Adempas riociguat Pulmonary Vasodilator Adlyxin lixisenatide Type 2 Diabetes Advocate Test Strips blood glucose test strips Blood Glucose Test Strips Aerospan** flunisolide Corticosteroids (Inhaled) Afrezza insulin Insulin (inhaled) Ampyra dalfampridine Multiple Sclerosis Agent Altoprev** lovastatin Cholesterol Alvesco** ciclesonide Corticosteroids -

The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy
Review Neuromuscular Diseases Early Diagnosis and Treatment – The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy Eugenio Mercuri,1 Ros Quinlivan2 and Sylvie Tuffery-Giraud3 1. Catholic University, Rome, Italy; 2. Great Ormond Street Hospital and National Hospital for Neurology and Neurosurgery, London, UK; 3. Laboratory of Genetics of Rare Diseases (LGMR), University of Montpellier, Montpellier, France DOI: https://doi.org/10.17925/ENR.2018.13.1.31 he understanding of the natural history of Duchenne muscular dystrophy (DMD) is increasing rapidly and new treatments are emerging that have the potential to substantially improve the prognosis for patients with this disabling and life-shortening disease. For many, Thowever, there is a long delay between the appearance of symptoms and DMD diagnosis, which reduces the possibility of successful treatment. DMD results from mutations in the large dystrophin gene of which one-third are de novo mutations and two-thirds are inherited from a female carrier. Roughly 75% of mutations are large rearrangements and 25% are point mutations. Certain deletions and nonsense mutations can be treated whereas many other mutations cannot currently be treated. This emphasises the need for early genetic testing to identify the mutation, guide treatment and inform genetic counselling. Treatments for DMD include corticosteroids and more recently, ataluren has been approved in Europe, the first disease-modifying therapy for treating DMD caused by nonsense mutations. The use of ataluren in DMD is supported by positive results from phase IIb and phase III studies in which the treatment produced marked improvements in the 6-minute walk test, timed function tests such as the 10 m walk/run test and the 4-stair ascent/descent test compared with placebo. -

Are Speci C Symptoms and Signs That Will Enable the Clinician to Recognise Disease Patterns That Will Narrow Down the Differenti
CHILDHOOD DEVELOPMENTAL SCREENING 2020 https://doi.org/10.33591/sfp.46.5.u5 UNIT NO. 5 Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street CHILDHOOD NEUROMUSCULAR DISORDERS examiner’s hands under the armpits and assessed for any arm periodic paralysis) and the rate of progression. An acute or delay/hypotonia, neuroimaging with MRI brain and aordable gene panels targeting neuromuscular disorders promising, with near-normal motor development in some receptor but reduced transcriptional activation and therefore 3. Orthopaedic Care neuromuscular diseases has resulted in faster and more accurate slip sign, and an attempt should be made to see if the infant can subacute onset of symptoms may be suggestive of an endocrine appropriate chromosomal/genetic testing can be considered. which are faster and less costly than whole exome or whole infants. is has resulted in new recommendations for neonatal has less side eects of other steroids. Clinical trials are still Prevention of contractures may be aected with appropriate diagnosis. Furthermore, new treatments are in the pipeline or N. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointes- A/Prof Stacey Tay Kiat Hong bear weight on both legs. e infant is then put into ventral or inammatory myositis, the chronic onset of symptoms may 2. If the child has evidence of lower limb involvement and genome sequencing. e choice of whole exome or whole newborn screening of SMA as well as a treatment algorithm for underway for head-to-head comparison of Vamorolone and resting splints of the ankles. Proper seating is also key to are already available for certain neuromuscular disorders, and 11 20 tinal and nutritional management. -

PRAC Draft Agenda of Meeting 14-17 March 2016
14 March 2016 EMA/PRAC/199507/2016 Corr.∗ Procedure Management and Committees Support Division Pharmacovigilance Risk Assessment Committee (PRAC) Draft agenda for the meeting on 14-17 March 2016 Chair: June Raine – Vice-Chair: Almath Spooner 14 March 2016, 13:00 – 19:00, room 3/A 15 March 2016, 08:30 – 19:00, room 3/A 16 March 2016, 08:30 – 19:00, room 3/A 17 March 2016, 08:30 – 16:00, room 3/E Organisational, regulatory and methodological matters (ORGAM) 31 March 2016, 10:00 - 12:00, room 7/B, via Adobe Connect Health and safety information In accordance with the Agency’s health and safety policy, delegates are to be briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, this agenda is a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. -

Animal Models of Duchenne Muscular Dystrophy: from Basic Mechanisms to Gene Therapy Joe W
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 195-213 doi:10.1242/dmm.018424 REVIEW Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy Joe W. McGreevy1, Chady H. Hakim1, Mark A. McIntosh1 and Dongsheng Duan1,2,* ABSTRACT The identification of the disease-causing gene and the molecular Duchenne muscular dystrophy (DMD) is a progressive muscle- basis for the DMD and BMD phenotypes establishes the foundation wasting disorder. It is caused by loss-of-function mutations in the for DMD gene therapy (Fig. 2A). To mitigate muscle disease, one dystrophin gene. Currently, there is no cure. A highly promising can either restore the full-length transcript or express a truncated but therapeutic strategy is to replace or repair the defective dystrophin in-frame dystrophin gene (Duan, 2011; Goyenvalle et al., 2011; gene by gene therapy. Numerous animal models of DMD have been Konieczny et al., 2013; Mendell et al., 2012; Verhaart and Aartsma- developed over the last 30 years, ranging from invertebrate to large Rus, 2012). Several gene therapy strategies are currently under mammalian models. mdx mice are the most commonly employed development. They include replacing the mutated gene with a models in DMD research and have been used to lay the groundwork functional candidate gene (gene replacement) or repairing the for DMD gene therapy. After ~30 years of development, the field has defective gene by targeted correction and exon skipping (gene reached the stage at which the results in mdx mice can be validated repair). Currently, adeno-associated virus (AAV)-mediated gene and scaled-up in symptomatic large animals. -
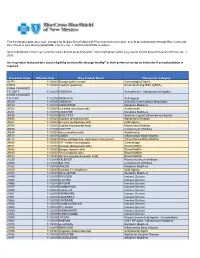
Procedure Code Effective Date Drug Product Name* Therapeutic
This list includes procedure code changes for Medical Benefit Specialty Pharmacy that may require benefit preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM) effective Jan. 1, 2020 for BCBSNM members. Items highlighted in blue may currently require benefit preauthorization. Items highlighted yellow may require benefit preauthorization effective Jan. 1, 2020. It is imperative that providers check eligibility and benefits through Availity® or their preferred vendor to determine if preauthorization is required. Procedure Code Effective Date Drug Product Name* Therapeutic Category 90378 1/1/2020 Synagis (palivizumab) Immunological Agent C9036 1/1/2020 Onpattro (patisiran) Small interfering RNA (siRNA) C9466 CHANGED TO J0517 1/1/2019 FASENRA Antiasthmatic - Monoclonal Antibodies C9493 CHANGED TO J1301 1/1/2019 RADICAVA ALS Agents J0129 1/1/2019 ORENCIA Selective Costimulation Modulators J0180 1/1/2019 FABRAZYME Metabolic Modifiers J0202 1/1/2020 Lemtrada (alemtuzumab) Autoimmune J0221 1/1/2019 LUMIZYME Metabolic Modifiers J0490 1/1/2019 BENLYSTA Systemic Lupus Erythematosus Agents J0565 1/1/2020 Zinplava (bezlotoxumab) Monoclonal Antibody J0567 1/1/2020 Brineura (cerliponase alfa) CNS Agents J0584 1/1/2020 Crysvita (burosumab-twza) Monoclonal Antibody J0598 1/1/2019 CINRYZE Complement Inhibitors J0638 1/1/2020 Ilaris (canakinumab) Autoimmune J0717 1/1/2019 CIMZIA Inflammatory Bowel Agents J0775 1/1/2020 Xiaflex (collagenase, clostridium histolyticum) Tissue Permeability Modifier J0800 1/1/2020 H.P. Acthar (corticotropin) -

Annexes to the Annual Report of the European Medicines Agency 2014
Annexes to the annual report of the European Medicines Agency 2014 Table of contents Annex 1 – Members of the Management Board ............................................................................. 2 Annex 2 – Members of the Committee for Medicinal Products for Human Use ................................... 4 Annex 3 – Members of the Pharmacovigilance Risk Assessment Committee ...................................... 6 Annex 4 – Members of the Committee for Medicinal Products for Veterinary Use ............................... 8 Annex 5 – Members of the Committee on Orphan Medicinal Products ............................................ 10 Annex 6 – Members of the Committee on Herbal Medicinal Products .............................................. 12 Annex 07 – Committee for Advanced Therapies .......................................................................... 14 Annex 8 – Members of the Paediatric Committee ........................................................................ 16 Annex 9 – Working parties and working groups .......................................................................... 18 Annex 10 – CHMP opinions in 2014 on medicinal products for human use ...................................... 22 Annex 11 – CVMP opinions in 2014 on medicinal products for veterinary use .................................. 36 Annex 12 – COMP opinions in 2014 on designation of orphan medicinal products ............................ 41 Annex 13 – HMPC European Union herbal monographs in 2014.................................................... -

Innovative Therapeutic and Delivery Approaches Using Nanotechnology to Correct Splicing Defects Underlying Disease
fgene-11-00731 July 14, 2020 Time: 11:18 # 1 REVIEW published: 14 July 2020 doi: 10.3389/fgene.2020.00731 Innovative Therapeutic and Delivery Approaches Using Nanotechnology to Correct Splicing Defects Underlying Disease Marc Suñé-Pou1†, María J. Limeres2†, Cristina Moreno-Castro3, Cristina Hernández-Munain4, Josep M. Suñé-Negre1, María L. Cuestas2 and Carlos Suñé3* 1 Drug Development Service (SDM), Faculty of Pharmacy, University of Barcelona, Barcelona, Spain, 2 Institute of Research in Microbiology and Medical Parasitology (IMPaM), Faculty of Medicine, University of Buenos Aires-CONICET, Buenos Aires, Argentina, 3 Department of Molecular Biology, Institute of Parasitology and Biomedicine “López-Neyra” (IPBLN-CSIC), Granada, Spain, 4 Department of Cell Biology and Immunology, Institute of Parasitology and Biomedicine “López-Neyra” (IPBLN-CSIC), Granada, Spain Alternative splicing of pre-mRNA contributes strongly to the diversity of cell- and tissue- Edited by: specific protein expression patterns. Global transcriptome analyses have suggested Rosanna Asselta, Humanitas University, Italy that >90% of human multiexon genes are alternatively spliced. Alterations in the Reviewed by: splicing process cause missplicing events that lead to genetic diseases and pathologies, Dario Balestra, including various neurological disorders, cancers, and muscular dystrophies. In recent University of Ferrara, Italy Mauricio Fernando Budini, decades, research has helped to elucidate the mechanisms regulating alternative University of Chile, Chile splicing -

Curriculum Vitae
Curriculum Vitae PERSONAL INFORMATION Ulrike Schara WORK EXPERIENCE 1991- 1997 Education in Paediatrics Paediatric Outpatient Centre,City Hospital Gelsenkirchen. (Germany) Paediatric Outpatient Centre. City Hospital Gelsenkirchen.<br/>Childrens Hospital, Ruhr University Bochum<br/> 1998- 2002 Senior Neuropediatrics Children's University Hospital Bochum (Germany) . 2002- 2003 Head of Neuropediatrics Children's University Hospital Bochum (Germany) 2004- 2006 Head of Neuropediatrics Children's Hospital, City of Neuss (Germany) 2007- Present Head of the Neuropaediatric Outpatient Centre and Consultant for Neuropaediatrics University of Essen (Germany) EDUCATION AND TRAINING 1991- 2012 MD, Professor Ruhr University of Bochum, Germany University of Essen (Germany) ADDITIONAL INFORMATION Expertise Publications Publcations of the past 5 years 1.Rudnik-Schöneborn S, Tölle D, Senderek J, Eggermann K, Elbracht M, Kornak U, von der Hagen M, Kirschner J, Leube B, Müller-Felber W, Schara U, von Au K, Wieczorek D, Bußmann C, Zerres K. Diagnostic algorithms in Charcot-Marie-Tooth neuropathies: experiences from a German genetic laboratory on the basis of 1206 index patients. Clin Genet. 2016 Jan;89(1):34-43 2.Byrne S, Jansen L, U-King-Im JM, Siddiqui A, Lidov HG, Bodi I, Smith L, Mein R, Cullup T, Dionisi-Vici C, Al-Gazali L, Al-Owain M, Bruwer Z, Al Thihli K, El-Garhy R, Flanigan KM, Manickam K, Zmuda E, Banks W, Gershoni-Baruch R, Mandel H, Dagan E, Raas-Rothschild A, Barash H, Filloux F, Creel D, Harris M, Hamosh A, Kölker S, Ebrahimi-Fakhari D, Hoffmann GF, Manchester D, Boyer PJ, Manzur AY, Lourenco CM, Pilz DT, Kamath A, Prabhakar P, Rao VK, Rogers RC, Ryan MM, Brown NJ, McLean CA, Said E, Schara U, Stein A, Sewry C, Travan L, Wijburg FA, Zenker M, Mohammed S, Fanto M, Gautel M, Jungbluth H. -

Med Pharm PA List to Build Pdfs.Xlsx
The following medications, typically administered in a provider's office or ambulatory/outpatient infusion center, require authorization prior to utilization. Authorization requests can be submitted via the Portal. Additionally, you are welcome to call our member services line to initiate the Prior Authorization process. This list is updated quaterly. Effective date: 1/1/2021 Therapeutic category Brand Name Generic Name HCPCS Immunologic agent Orencia, IV infusion abatacept J0129 Botulinum toxins Dysport abobotulinumtoxin B J0586 HER-2 receptor drug Kadcyla ado-trastuzumab emtansine J9354 Ophthalmic injectable Eylea afilbercept J0178 Enzyme replacement drug Fabrazyme agalsidase beta J0180 Multiple sclerosis drug Lemtrada alemtuzumab J0202 Enzyme replacement drug Lumizyme alglucosidase alfa J0221 Enzyme replacement drug Strensiq asfotase alfa J3590 (non-specific) PD1-PDL1 drug Tecentriq atezolizumab J9022 PD1-PDL1 drug Bavencio avelumab J9023 CAR-T Therapy Yescarta axicabtageneciloleucel inpatient admin Systemic lupus erythematosus (SLE) drug Benlysta belimumab J0490 Antineoplastic agent Beleodaq belinostat J9032 Antineoplastic Belrapzo bendamustine HCl J9036 Antineoplastic Bendeka bendamustine HCl J9034 Antineoplastic agent Treanda bendamustine HCl J9033 Respiratory injectable Fasenra benralizumab J0517 J9035/J3590 (ophthalmology Antineoplastic Avastin bevacizumab uses this non-specific code) Antineoplastic agent Mvasi bevacizumab-awwb Q5107 Antineoplastic agent Zirabev bevacizumab-bvzr Q5118 Antineoplastic agent Blincyto blinatumomab