Mating Systems of Blood-Feeding Flies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Function of Copulatory Plugs in House Mice: Mating Behaviour and Paternity
1 Function of copulatory plugs in house mice: mating behaviour and paternity 2 outcomes of rival males 3 4 Andreas Sutter1,3, Leigh W. Simmons2, Anna K. Lindholm1 and Renée C. Firman2 5 6 1Institute of Evolutionary Biology and Environmental Studies, University of Zurich, 7 Winterthurerstrasse 190, 8057 Zurich, Switzerland. 8 2Centre for Evolutionary Biology, School of Animal Biology (M092), The University of Western 9 Australia, Crawley, 6009, Australia. 10 3 E-mail:[email protected] 11 12 Corresponding author: Andreas Sutter, IEU – Animal Behavior, Winterthurerstrasse 190, 8057 13 Zürich, Switzerland; Tel: +41 79 545 9015; email: [email protected]; 14 15 Abbreviated title: Function of copulatory plugs 16 1 17 Abstract 18 Polyandry is widespread across animal taxa, and subjects males to intense post-copulatory sexual 19 selection which favors adaptations that enhance a male’s paternity success, either by decreasing the 20 risk of sperm competition and/or by increasing the competitiveness of the ejaculate. Copulatory 21 plugs deposited by males are thought to have evolved in the context of sperm competition. 22 However, experimental studies that assess the function of copulatory plugs remain scarce. 23 Moreover, most studies have used unnatural manipulations, such as ablating plug-producing male 24 glands or interrupting copulations. Here, we investigated whether repeated ejaculation affects plug 25 size in a mammalian model species, the house mouse. When males experience short periods of 26 sexual rest we found that plug size decreased over repeated ejaculations so that time since last 27 ejaculation can be applied as an approximation for plug size. -

Identification Key for Mosquito Species
‘Reverse’ identification key for mosquito species More and more people are getting involved in the surveillance of invasive mosquito species Species name used Synonyms Common name in the EU/EEA, not just professionals with formal training in entomology. There are many in the key taxonomic keys available for identifying mosquitoes of medical and veterinary importance, but they are almost all designed for professionally trained entomologists. Aedes aegypti Stegomyia aegypti Yellow fever mosquito The current identification key aims to provide non-specialists with a simple mosquito recog- Aedes albopictus Stegomyia albopicta Tiger mosquito nition tool for distinguishing between invasive mosquito species and native ones. On the Hulecoeteomyia japonica Asian bush or rock pool Aedes japonicus japonicus ‘female’ illustration page (p. 4) you can select the species that best resembles the specimen. On japonica mosquito the species-specific pages you will find additional information on those species that can easily be confused with that selected, so you can check these additional pages as well. Aedes koreicus Hulecoeteomyia koreica American Eastern tree hole Aedes triseriatus Ochlerotatus triseriatus This key provides the non-specialist with reference material to help recognise an invasive mosquito mosquito species and gives details on the morphology (in the species-specific pages) to help with verification and the compiling of a final list of candidates. The key displays six invasive Aedes atropalpus Georgecraigius atropalpus American rock pool mosquito mosquito species that are present in the EU/EEA or have been intercepted in the past. It also contains nine native species. The native species have been selected based on their morpho- Aedes cretinus Stegomyia cretina logical similarity with the invasive species, the likelihood of encountering them, whether they Aedes geniculatus Dahliana geniculata bite humans and how common they are. -

Zootaxa, New Records of Haemagogus
Zootaxa 1779: 65–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) New records of Haemagogus (Haemagogus) from Northern and Northeastern Brazil (Diptera: Culicidae, Aedini) JERÔNIMO ALENCAR1, FRANCISCO C. CASTRO2, HAMILTON A. O. MONTEIRO2, ORLANDO V. SILVA 2, NICOLAS DÉGALLIER3, CARLOS BRISOLA MARCONDES4*, ANTHONY E. GUIMARÃES1 1Laboratório de Diptera, Departamento de Entomologia, Instituto Oswaldo Cruz, Av. Brasil 4365, CEP: 21045-900 Manguinhos, Rio de Janeiro RJ, Brazil. 2Laboratório de Arbovírus, Instituto Evandro Chagas, Av. Almirante Barroso 492, CEP: 66090-000, Belém, PA, Brazil. 3Institut de Recherche pour le Développement (IRD-UMR182), LOCEAN-IPSL, case 100, 4 Place Jussieu, 75252 Paris Cedex 05, France 4 Departamento de Microbiologia e Parasitologia, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, 88040- 900 Florianópolis, Santa Catarina, Brazil Haemagogus (Haemagogus) is restricted mostly to the Neotropical Region, including Central America, South America and islands (Arnell, 1973). Of the 24 recognized species of this subgenus, 15 occur in South America, including the Anti- lles. However, the centre of distribution of the genus Haemagogus is Central America, where 19 of the 28 species (including four species of the subgenus Conopostegus Zavortink [1972]) occur (Arnell, 1973). Haemagogus (Hag.) includes species with great significance as vectors of Yellow Fever (YF) virus and other arbovi- rus, both experimentally (Waddell, 1949) and in the field (Vasconcelos, 2003). During entomological surveys from 1982 to 2004, the Arbovirus Laboratory of Evandro Chagas Institute obtained specimens of Haemagogus from several localities not reported in the literature. New records are listed in Table 1 and study localities shown on Figure 1. -

Sperm Competition and Male Social Dominance in the Bank Vole (Myodes Glareolus)
SPERM COMPETITION AND MALE SOCIAL DOMINANCE IN THE BANK VOLE (MYODES GLAREOLUS) Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Jean-Fran^ois Lemaitre 1 Table of contents List of Tables.......................................................................................................................................6 List of Figures.....................................................................................................................................8 Declaration of work conducted.................................................................................................... 10 Abstract ......................................................................................................................................13 Chapter 1: General introduction............................................................................................... 15 1.1 Chapter overview........................................................................................................... 15 1.2 Sexual selection..... .......................................................................................................... 15 (a) Sexual selection............................................................................................................... 15 (b) Sexual selection and sex-roles........................................................................................16 (c) Pre-copulatory sexual selection......................................................................................18 -

Diptera: Culicidae: Aedini) Into New Geographic Areas
European Mosquito Bulletin, 27 (2009), 10-17. Journal of the European Mosquito Control Association ISSN 1460-6127; w.w.w.e-m-b.org First published online 1 October 2009 Recent introductions of aedine species (Diptera: Culicidae: Aedini) into new geographic areas John F. Reinert Center for Medical, Agricultural and Veterinary Entomology (CMAVE), United States Department of Agriculture, Agricultural Research Service, 1600/1700 S.W. 23rd Drive, Gainesville, FL 32608-1067, USA, Email: [email protected]. Abstract Information on introductions to new geographic areas of species in the aedine generic-level taxa Aedimorphus, Finlaya, Georgecraigius, Halaedes, Howardina, Hulecoeteomyia, Rampamyia, Stegomyia, Tanakaius and Verrallina is provided. Key words: Aedimorphus, Finlaya, Georgecraigius atropalpus, Halaedes australis, Howardina bahamensis, Hulecoeteomyia japonica japonica, Rampamyia notoscripta, Stegomyia aegypti, Stegomyia albopicta, Tanakaius togoi, Verrallina Introduction World. Dyar (1928), however, notes that there are no nearly related species in As indicated in the series of papers on the American continent, but many such the phylogeny and classification of in the Old World, especially in Africa, mosquitoes in tribe Aedini (Reinert et and he considered that it was probably al., 2004, 2006, 2008), some aedine the African continent from which the species have been introduced into new species originated”. Christophers also geographical areas in recent times. noted that “The species is almost the Species of Aedini found outside of their only, if not the only, mosquito that, with natural ranges are listed below with their human agency, is spread around the literature citations. whole globe. But in spite of this wide zonal diffusion its distribution is very Introductions of Aedine Species to strictly limited by latitude and as far as New Areas present records go it very rarely occurs beyond latitudes of 45o N. -

Copulatory Plugs Inhibit the Reproductive Success of Rival Males
doi: 10.1111/jeb.12956 Copulatory plugs inhibit the reproductive success of rival males R. MANGELS1 ,K.TSUNG1 ,K.KWAN&M.D.DEAN Molecular and Computational Biology, University of Southern California, Los Angeles, CA, USA Keywords: Abstract copulatory plug; Ejaculated proteins play important roles in reproductive fitness. In many mate guarding; species, seminal fluid coagulates and forms what has been referred to as a sperm competition. copulatory plug in the female’s reproductive tract. In mice, previous work demonstrated that knockout males missing a key seminal fluid protein were unable to form a plug and less successful at siring litters in noncompetitive matings (one female, one male), probably the result of reduced sperm trans- port or insufficient stimulation of the female. Here, we extend these previ- ous studies to competitive matings (one female, two males) and make two key insights. First, when first males were unable to form a plug, they lost almost all paternity to second males to mate. Thus, the copulatory plugs of second males could not rescue the reduced fertility of first males. Second, we showed that the copulatory plug of first males effectively blocked fertil- ization by second males, even if first males were vasectomized. Taken together, our experiments demonstrated that first males lost almost all paternity if they never formed a plug. We discuss our results in the context of natural populations, where in spite of the strong effects seen here, preg- nant female mice regularly carry litters fertilized by more than one male. Masumoto, 1993; Uhl et al., 2010), insects (Parker, 1970; Introduction Dickinson & Rutowski, 1989; Orr & Rutowski, 1991; Characterizing the function of ejaculated proteins is Rogers et al., 2009), reptiles (Devine, 1975; Herman, critical to a comprehensive understanding of reproduc- 1994; Friesen et al., 2013) and mammals (Devine, 1975; tive health and evolutionary fitness. -

Classification of Mosquitoes in Tribe Aedini 925
FORUM Classification of Mosquitoes in Tribe Aedini (Diptera: Culicidae): Paraphylyphobia, and Classification Versus Cladistic Analysis HARRY M. SAVAGE Centers for Disease Control and Prevention, P.O. Box 2087, Fort Collins, CO 80522 J. Med. Entomol. 42(6): 923Ð927 (2005) Downloaded from https://academic.oup.com/jme/article/42/6/923/886357 by guest on 29 September 2021 ABSTRACT Many mosquito species are important vectors of human and animal diseases, and others are important nuisance species. To facilitate communication and information exchange among pro- fessional groups interested in vector-borne diseases, it is essential that a stable nomenclature be maintained. For the Culicidae, easily identiÞable genera based on morphology are an asset. Major changes in generic concept, the elevation of 32 subgenera within Aedes to generic status, and changes in hundreds of species names proposed in a recent article demand consideration by all parties interested in mosquito-borne diseases. The entire approach to Aedini systematics of these authors was ßawed by an inordinate fear of paraphyletic taxa or Paraphylyphobia, and their inability to distinguish between classiÞcation and cladistic analysis. Taxonomists should refrain from making taxonomic changes based on preliminary data, and they should be very selective in assigning generic names to only the most important and well-deÞned groups of species. KEY WORDS Aedini classiÞcation, Aedes, Ochlerotatus, paraphylyphobia, mosquito classiÞcation MANY MOSQUITO SPECIES IN the tribe Aedini, a cosmo- In this communication, I brießy review taxonomic politan group represented by 11 genera and Ϸ1,239 categories in a zoological classiÞcation, the Interna- species, are important vectors of human and animal tional Code of Zoological Nomenclature (the Code), diseases, and many others are of considerable eco- the development of generic concept within the Cu- nomic importance as nuisance or pest species. -

A Multiplex PCR Assay for Six Aedini Species, Including Aedes Albopictus
A Multiplex PCR Assay for Six Aedini Species, Including Aedes albopictus Woo Jun Bang Kyungpook National University College of Natural Sciences Min Hyeok Won Kyungpook National University College of Natural Sciences Seong Tae Cho Kyungpook National University College of Natural Sciences Jihun Ryu Kyungpook National University College of Natural Sciences Kwang Shik Choi ( [email protected] ) Kyungpook National University, Daehak-ro 80, Daegu Methodology Keywords: Aedini species, Aedes albopictus, Internal transcribed spacer 2(ITS2), multiplex PCR assay Posted Date: December 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-127286/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Parasites & Vectors on July 28th, 2021. See the published version at https://doi.org/10.1186/s13071-021-04871-7. Page 1/8 Abstract Background Mosquitoes, as vectors of various human pathogens, are signicant drivers of serious human illness. In particular, those species in the Aedini tribe, which typically transmit dengue virus, Chikungunya fever virus, and Zika virus, are increasing their range because of climate change and international commerce. In order to prevent mosquito-borne disease, accurate mosquito species identication and monitoring are needed. The goal of this work was to develop a rapid and simple molecular diagnostic method for six morphologically similar Aedini species (Aedes avopictus, Aedes albopictus, Ochlerotatus koreicus, Ochlerotatus japonicus, Ochlerotatus togoi, and Ochlerotatus hatorii) in Korea. Methods In total, 109 samples were used in this study. The internal transcribed spacer 2 (ITS2) regions from all six species were amplied, sequenced and analyzed using Mega 6. -

The Taxonomic History of Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae)
insects Article The Taxonomic History of Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae) Lílian Ferreira de Freitas * and Lyric C. Bartholomay Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA; [email protected] * Correspondence: [email protected] Simple Summary: Mosquitoes are an extremely diverse group of aquatic insects, distributed over all continents except Antarctica. The mosquitoes belonging to the subgenus called Ochlerotatus, as understood by Reinert et al., comprise a group of several species which are endemic to the Americas, some of which are important vectors of human and animal pathogens. However, this group is characteristically undefined, i.e., no sets of characters define the group, and this presents a major challenge for the understanding of the evolutionary tree of the mosquitoes. This work underscores and contextualizes the complex taxonomic history of the group, reveals the major challenges we face in order to resolve the definition of this group, and presents a path forward for its successful revision. Abstract: A review of all taxonomic actions within the subgenus Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae) sensu Reinert et al. (2008) is provided. In particular, the complex historical taxo- nomic treatment of the type species of this group is dissected and explained in detail. Additionally, current challenges with the definition of the subgenus and its constituents are discussed, as are the requisite steps for a successful revision of the taxon. Going forward, we conclude that a taxonomic Citation: Ferreira de Freitas, L.; revision of the species should include a neotype designation for Ochlerotatus scapularis (Rondani, Bartholomay, L.C. -

Do the Size and Age of Mating Plugs Alter Their Efficacy in Protecting Paternity?
Behav Ecol Sociobiol (2014) 68:1321–1328 DOI 10.1007/s00265-014-1742-7 ORIGINAL PAPER Do the size and age of mating plugs alter their efficacy in protecting paternity? Katrin Kunz & Melanie Witthuhn & Gabriele Uhl Received: 19 November 2013 /Revised: 28 April 2014 /Accepted: 9 May 2014 /Published online: 10 June 2014 # Springer-Verlag Berlin Heidelberg 2014 Abstract An obvious means to secure paternity is the pro- of theoretical considerations on the evolution of effective duction of a mating plug that blocks the female genital open- mating plugs. ing after mating. Although the mechanical efficacy and per- sistence of plugs on/in the female genital openings are key Keywords Mating plug . Sexual selection . Sperm traits that determine the degree of paternity protection, these competition . Monopolization . Paternity protection . Dwarf factors have hardly been explored. We therefore investigated spider . Erigoninae the influence of the size of the amorphous plug material (experimentally terminated mating duration as a proxy) and age of the mating plug (time interval between copulations with Introduction two successive males) on the efficacy of the plug by analysing the mating success of subsequent males in the dwarf spider In polyandrous species, post-copulatory male-male competi- Oedothorax retusus (Linyphiidae: Erigoninae). Overall, sub- tion occurs if sperm of different males are stored within the sequent males attempted to mate in 82 % of trials but only female genital tract (Parker 1970). Polyandry is widespread in 32.5 % of these resulted in copulation, demonstrating that the various animal taxa and has led to numerous behavioural, plugs are effective safeguards against remating. Remating physiological and morphological male adaptations against probability was significantly higher after previous short cop- sperm competition (Birkhead and Møller 1998;Parker1998; ulations (~small plug size) compared to long copulations Simmons 2001; Eberhard 2009). -

Mating Strategies and Resulting Patterns in Mate Guarding Crustaceans : an Empirical and Theoretical Approach Matthias Galipaud
Mating strategies and resulting patterns in mate guarding crustaceans : an empirical and theoretical approach Matthias Galipaud To cite this version: Matthias Galipaud. Mating strategies and resulting patterns in mate guarding crustaceans : an empir- ical and theoretical approach. Reproductive Biology. Université de Bourgogne, 2012. English. NNT : 2012DIJOS111. tel-01124096 HAL Id: tel-01124096 https://tel.archives-ouvertes.fr/tel-01124096 Submitted on 6 Mar 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université de Bourgogne UMR CNRS 6282 Biogéosciences THÈSE Pour l’obtention du grade de Docteur de l’Université de Bourgogne Discipline : Sciences de la Vie Spécialité : Ecologie Evolutive Mating strategies and resulting patterns in mate guarding crustaceans: an empirical and theoretical approach Matthias Galipaud Directeur de thèse : Loïc Bollache Co-directeur de thèse : François-Xavier Dechaume-Moncharmont Jury Loïc Bollache, Professeur, Université de Bourgogne Directeur Frank Cézilly, Professeur, Université de Bourgogne Examinateur François-Xavier -
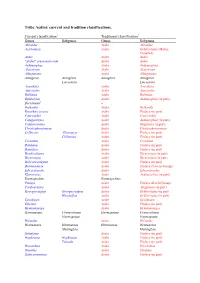
Tribe Aedini: Current and Tradition Classifications
Tribe Aedini: current and tradition classifications. Current classification1 Traditional classification2 Genus Subgenus Genus Subgenus Abraedes Aedes Abraedes Acartomyia Aedes Ochlerotatus (Mariae Complex) Aedes Aedes Aedes “Aedes” sensu auctorum Aedes Aedes Aedimorphus Aedes Aedimorphus Alanstonea Aedes Alanstonea Albuginosus Aedes Albuginosus Armigeres Armigeres Armigeres Armigeres Leicesteria Leicesteria Ayurakitia Aedes Ayurakitia Aztecaedes Aedes Aztecaedes Belkinius Aedes Belkinius Bifidistylus Aedes Aedimorphus (in part) Borichinda3 – Bothaella Aedes Bothaella Bruceharrisonius Aedes Finlaya (in part) Cancraedes Aedes Cancraedes Catageiomyia Aedes Aedimorphus (in part) Catatassomyia Aedes Stegomyia (in part) Christophersiomyia Aedes Christophersiomyia Collessius Alloeomyia Aedes Finlaya (in part) Collessius Aedes Finlaya (in part) Cornetius Aedes Cornetius Dahliana Aedes Finlaya (in part) Danielsia Aedes Finlaya (in part) Dendroskusea Aedes Diceromyia (in part) Diceromyia Aedes Diceromyia (in part) Dobrotworskyius Aedes Finlaya (in part) Downsiomyia Aedes Finlaya (Niveus Group) Edwardsaedes Aedes Edwardsaedes Elpeytonius Aedes Aedimorphus (in part) Eretmapodites Eretmapodites Finlaya Aedes Finlaya (Kochi Group) Fredwardsius Aedes Stegomyia (in part) Georgecraigius Georgecraigius Aedes Ochlerotatus (in part) Horsfallius Aedes Ochlerotatus (in part) Geoskusea Aedes Geoskusea Gilesius Aedes Finlaya (in part) Gymnometopa Aedes Gymnometopa Haemagogus Conopostegus Haemagogus Conopostegus Haemagogus Haemagogus Halaedes Aedes Halaedes Heizmannia