Cupressus Torulosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cupressus Torulosa Is a Good Substitute of Two Selected Juniperus Species for Aroma Potentials Hema Lohani, Nirpendra Kumar Chauhan and Harish Ch
Arom & at al ic in P l ic a n d t Lohani et al., Med Aromat Plants 2013, 2:2 e s M Medicinal & Aromatic Plants DOI: 0.4172/2167-0412.1000122 ISSN: 2167-0412 ResearchLetter Article OpenOpen Access Access Cupressus torulosa is a Good Substitute of Two Selected Juniperus Species for Aroma Potentials Hema Lohani, Nirpendra Kumar Chauhan and Harish Ch. Andola* Centre for Aromatic Plants (CAP) Industrial Estate, Selaqui-248197,Dehradun (Uttarakhand), India Essential oils which are complex mixture of terpenoids and other survival of the Juniperus species at a risk in nature due to continuous class of components isolated from different plant parts of aromatic plants, exploration in for preparation of tradition incenses and material for find impotent place in industry due to extensive uses in food, flavour, essential oil extraction etc. essential oil analysis of the needles and fragrances and pharmaceutical industry, More than 250 different type of berry of Juniperus species i.e. J. communis, contain monoterpene essential oil worth US$ 1.2 billion per annum are traded in the globe [1] hydrocarbons (76.2-81.4%). Major components such as α-pinene A number of countries produced different kind of essential oils. India (31.8-49.5%) and limonene (13.7-19.5%) δ-3-carene (9.7%- 14.7%) ranks second in world trade due to wide application in cosmetic, food, sabinene (0.8-6.7%), β-myrcene (2.4-5.6%), β-pinene (2.1-4.3%) and and pharmaceutical, therapeutically these used as antiseptic, stimulant, α-terpinyl acetate (1.7-2.9%). -

Cypress Species Choice and Minimising the Risk of Canker
GROWING CYPRESSES FOR TIMBER Species choice and minimising the risk of canker Information Note 1 Cypresses have long been a favourite alternative to radiata pine for New Zealand’s farm foresters, small-scale plantation owners, and some large-scale growers. IN GENERAL, CYPRESSES: • Are a versatile species with proven performance both as a timber producer and as a shelter species. • Produce versatile timber of relatively high value (compared with radiata pine) that is very easy to saw and dry, and has many end uses. • Grow best on moderately fertile, well- drained, sheltered sites, but with careful species and genotype (seedlot) selection will perform well on a reasonably wide range of sites. • Produce their best timber on sheltered sites. 18-year old Cupressus macrocarpa. • Produce quality timber even as young trees, unlike radiata pine. • Have a reputation of being prone to canker. However this is chiefly a problem of C. macrocarpa, and Leyland cypresses on warmer and exposed sites. Susceptibility to canker depends on site, species, and within any given species, the seedlot. Some seedlots are more resistant to canker than others of the same species. New more canker- resistant planting stock is becoming available. • Are prone to toppling on fertile sites, especially if poorly drained, and are particularly unstable on water-logged clay soils. • May cause abortion if foliage is eaten by pregnant cattle. Caution is advised. Cattle raised with access to cypresses tend not to eat the foliage. There is an established market for cypress timber based on ‘macrocarpa’ – a species widely planted throughout New Zealand for many decades, both in plantations and in farm shelter plantings. -

Gene Duplications and Genomic Conflict Underlie Major Pulses of Phenotypic 2 Evolution in Gymnosperms 3 4 Gregory W
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.13.435279; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Gene duplications and genomic conflict underlie major pulses of phenotypic 2 evolution in gymnosperms 3 4 Gregory W. Stull1,2,†, Xiao-Jian Qu3,†, Caroline Parins-Fukuchi4, Ying-Ying Yang1, Jun-Bo 5 Yang2, Zhi-Yun Yang2, Yi Hu5, Hong Ma5, Pamela S. Soltis6, Douglas E. Soltis6,7, De-Zhu Li1,2,*, 6 Stephen A. Smith8,*, Ting-Shuang Yi1,2,*. 7 8 1Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, 9 Kunming, Yunnan, China. 10 2CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of 11 Botany, Chinese Academy of Sciences, Kunming, China. 12 3Shandong Provincial Key Laboratory of Plant Stress Research, College of Life Sciences, 13 Shandong Normal University, Jinan, Shandong, China. 14 4Department of Geophysical Sciences, University of Chicago, Chicago, IL, USA. 15 5Department of Biology, Huck Institutes of the Life Sciences, Pennsylvania State University, 16 University Park, PA, USA. 17 6Florida Museum of Natural History, University of Florida, Gainesville, FL, USA. 18 7Department of Biology, University of Florida, Gainesville, FL, USA. 19 8Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, 20 MI, USA. 21 †Co-first author. 22 *Correspondence to: [email protected]; [email protected]; [email protected]. -

Two Distinct Himalayan Cypress Species Cupressus Tortulosa and Cupressus Cashmeriana with Additional Comparison to Cupressus Torulosa
Bull. CCP 3 (3): 99-115. (12.2014) D. Mаеrki Two distinct Himalayan cypress species Cupressus tortulosa and Cupressus cashmeriana with additional comparison to Cupressus torulosa In a previous article (Mаеrki 2013b), the trees grown in France, Italy and Switzerland under the label Cupressus cashmeriana Carrière were investigated. It was discovered that the cypresses grown in France are different from those cultivated in Italy, which better match the material collected by Griffith in Bhutan and described by him under the name Cupressus tortulosa. A summary of the main differences has been proposed (Mаеrki, 2013b: 49-50) together with analysis of Carrière’s protologue (1867) and discussion of the correct origin of the French plants in “Tibet” (now Arunachal Pradesh in India). The scope of the present article is to detail these differences with new observations and statistical data. Cupressus torulosa D.Don, Cupressus tortulosa and Cupressus cashmeriana are all growing west to 1 east on the southern slopes of the Himalaya in well separated distribution ranges ; west Nepal, and Himachal Pradesh and Uttarakhand in India, for C. torulosa, Bhutan for C. tortulosa and Arunachal Pradesh (India) for C. cashmeriana. All populations of each species are scattered in different valleys. It is quite likely that the compartmentalisation of these populations over several millions years allowed speciation processes from a common ancestor. There are also lots of planted trees near temples, monasteries and fortresses whose origins are not recorded. Several wild stands are very difficult to access, sometimes almost impossible above high cliffs, and are still in need of investigation; further taxa could possibly yet be discovered. -

Volume 25, No. 4 Southeastern Conifer
S. Horn Volume 25, no. 4 Southeastern Conifer Quarterly December 2018 American Conifer Society Southeast Region Alabama, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, West Virginia From the Southeast Region President As you can see from this photo, actvity in the Galloway’s garden has slowed down. Thus I have a good opportunity to provide a brief update on the ongoing Southeast Region initatves. In conjuncton with the Saturday, February 9 Atlanta Winter Natonal ACS Board Meetng, our Region will coordinate a Rendezvous at the Atlanta Botanical Garden on Sunday, February 10. Preliminary details were provided in a recent e-mail and a sign -up will be forthcoming. We hope you can join us. Work contnues on our annual SER Meetng to be held in October 2019. Please see the artcle from Director Jef Harvey regarding this event. Hopefully, this is a good tme of the year to kick back and enjoy the fall season. We’ll view some terrifc private gardens, have interestng dialogue, and enjoy ACS camaraderie. Complete informaton on the entre event will be sent as soon as the details are fnalized. Our reference garden program is now under new leadership. Welcome Beth Jimenez and Amelia Lane. They have taken the reins from Barbie Colvin as Co-Chairs. Barbie requested a break for extensive travel. We will be announcing Board electons afer the frst of the year. Electons for two-year terms of ofce are held annually, with ofces coming up for electon in alternate years. In 2019 we will elect a President and a Secretary/ Treasurer. -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

The Extent of Native Vegetation Across the Development Site and Location of Sampling Quadrats
Appendix 1 - Figures Greenwich Hospital Redevelopment DSF04 Coastal Enriched Sandstone Dry Forest equivalent PCT 1776 Smooth-barked Apple - Red Bloodwood open forest on enriched sandstone slopes around Sydney and the Central Coast DSF06 Coastal Sandstone Foreshores Forest equivalent PCT 1778 Smooth-barked Apple - Coast Banksia / Cheese Tree open forest on sandstone slopes on the foreshores of the drowned river valleys of Sydney RF02 Coastal Sandstone Gallery Rainforest equivalent PCT 1828 Coachwood - Lilly Pilly - Water Gum gallery rainforest in sandstone gullies of the Sydney basin Landscaped gardens (native/exotic) N Weed infestation Figure 17: The extent of native vegetation across the development site and location of sampling quadrats. Keystone Ecological 59 Ref: LCC 17-916 – January 2019 Appendix 1 - Figures Greenwich Hospital Redevelopment Figure 18: Foraging habitat for microbats and Pteropus poliocephalus Grey-headed Flying-fox within vegetation zone 1 that will be removed by the proposal is shown hatched. Keystone Ecological 60 Ref: LCC 17-916 – January 2019 Appendix 1 - Figures Greenwich Hospital Redevelopment Tree Species Fate 9 Ficus rubiginosa Port Jackson Fig Remove 15 Eucalyptus pilularis Blackbutt Keep 21 Eucalyptus pilularis Blackbutt Keep 24 Eucalyptus pilularis Blackbutt Keep 39 Eucalyptus pilularis Blackbutt Remove 48 Eucalyptus pilularis Blackbutt Keep 81 Angophora costata Smooth-barked Apple Keep 82 Corymbia citriodora Lemon-scented Gum Keep 151 Acer negundo Box Elder Maple Keep 163 Angophora costata Smooth-barked Apple Remove 177 Eucalyptus pilularis Blackbutt Remove Figure 19: Hollow-bearing tree locations, representing potential roost habitat for Myotis macropus Large-footed Myotis. Keystone Ecological 61 Ref: LCC 17-916 – January 2019 Appendix 1 - Figures Greenwich Hospital Redevelopment Figure 20: The extent of native vegetation impacted by the proposal and requiring offset (red), totalling 0.44 hectares of DSF04 / PCT 1776. -

Cupressus Tortulosa Griffith
45 THE TAXONOMY OF THE GRIFFITH CYPRESS (Cupressus tortulosa Griffith). by John Silba* Abstract Cupressus was first described from cultivated material collected near Roongdong, Bhutan by Griffith at 1920 m. elevation. Griffith (1848) described the plant as Cupressus pendula Griffith based on Griffith n°529. However, this herbarium collection was apparently lost or destroyed and cannot be located at present. Furthermore, the name Cupressus pendula was earlier published as Cupressus pendula Thunb. (1783), and is therefore invalid. Indeed, the older homonym Cupressus pendula Thunb. belongs to a quite different species altogether or Platycladus orientalis (L.) Franco. Realizing that his species name was invalid, Griffith renamed the Cupressus as Cupressus tortulosis Griffith (Griffith 1854-a). In the type description Griffith lists two herbarium collections, one from Roongdong and the other from Dewangiri (n°27). Since Griffith n°529 was earlier quoted as the type (holotype) for C. pendula Griffith, then Griffith n°27 from Dewangiri (K) must be proposed as the lectotype for Cupressus tortulosis Griffith. Farjon (2005) incorrectly lists Griffith n°27 (K) as the holotype of C. pendula Griff. In his monograph on the Cupressaceae Farjon attempts to cleverly and cavalierly dismiss the name Cupressus tortulosis Griff. as a mere synonym of Cupressus cashmeriana Carr. However, Farjon (2005) is clearly not clever, he is wrong and the name Cupressus tortulosis Griffith is rightfully the earliest valid name published for the cypress (Cupressus) of Bhutan. According to the ICBN therefore the name Cupressus tortulosis Griff. has precedence over the later name Cupressus cashmeriana Royle ex Carriere. Key words: Bhutan, Griffith, priority, nomenclature, lectotype, Cupressus cashmeriana, Cupressus tortulosa. -

Phloeosinus Cupressi, the Cypress Bark Beetle
Phloeosinus cupressi, the Cypress Bark Beetle Forest and Timber Insects in New Zealand No. 3 Revised 2009 Based on R. Zondag (1976) Insect: Phloeosinus cupressi Hopkins (Coleoptera: Scolytidae) Fig. 1 - Cypress barkbeetle, male. Arrow indicates incomplete rows of tubercules. Fig. 2 - Cypress barkbeetle, female. Arrows indicate complete rows of tubercules. Type of injury The cypress barkbeetle breeds under the bark of dead and dying trees but is more often found under the bark of felled trees or in dead branches. The adults feed on the inner bark and also, when newly emerged, bore into small twigs, mining down the centre and leaving only a thin shell. Such attacked twigs break off. Fig. 3 - Workings of the cypress barkbeetle under the bark of Cupressus macrocarpa. The larval tunnels spread out from the central gallery made by the parent beetles. Hosts In New Zealand this species has been recorded breeding and feeding in Cupressus macrocarpa, C. arizonica (Arizona cypress), C. benthamii (Bentham cypress), and Chamaecyparis lawsoniana (Lawson cypress), but twig boring only in Cupressus torulosa (Bhutan cypress); in California it has been recorded from Cupressus, Cryptomeria, Chamaecyparis, Thuja orientalis (Chinese arborvitae), T. plicata (western red cedar), Calocedrus decurrens (incense cedar), and Sequoia sempervirens (Coast redwood); and in Australia from Cupressus torulosa and Callitris (cypress pine), but these records do not always indicate whether this was breeding attack or twigboring. Distribution This barkbeetle from California has a wide distribution in the North Island but in the South Island it appears to be restricted to Nelson, Marlborough and Canterbury. Economic importance This insect is of minor importance as it does not kill trees or large branches. -

Identification Key to the Cypress Family (Cupressaceae)1
Feddes Repertorium 116 (2005) 1–2, 96–146 DOI: 10.1002/fedr.200411062 Weinheim, Mai 2005 Ruhr-Universität Bochum, Lehrstuhl für Spezielle Botanik, Bochum C. SCHULZ; P. KNOPF & TH. STÜTZEL Identification key to the Cypress family (Cupressaceae)1 With 11 Figures Summary Zusammenfassung The identification of Cupressaceae taxa, except for Bestimmungsschlüssel für die Familie der Cup- some local and easily distinguishable taxa, is diffi- ressaceae cult even for specialists. One reason for this is the lack of a complete key including all Cupressaceae Die Bestimmung von Cupressaceae-Taxa ist mit taxa, another reason is that diagnoses and descrip- Ausnahme einiger lokaler und leicht bestimmbarer tions are spread over several hundred publications Taxa schwierig, selbst für Spezialisten. Ein Grund, which are sometimes difficult to access. Based on warum es noch keinen vollständigen Bestimmungs- morphological studies of about 3/4 of the species and schlüssel mit allen Cupressaceae-Taxa gibt ist, dass a careful compilation of the most important descrip- die Sippen-Beschreibungen sich auf mehrere hundert tions of Cupressaceae, a first identification key for Publikationen verteilen, welche teilweise schwierig the entire Cypress family (Cupressaceae) could be zu beschaffen sind. Etwa 3/4 der Cupressaceae-Ar- set up. The key comprises any of the 30 genera, 134 ten wurden morphologisch untersucht und die wich- species, 7 subspecies, 38 varieties, one form and thus tigsten Beschreibungen zusammengefasst, daraus all 180 taxa recognized by FARJON (2001). The key wurde dann der erste vollständige Bestimmungs- uses mainly features of adult leaves, female cones schlüssel für Cupressaceae erstellt. Der Bestim- and other characters which are all relatively easy to mungsschlüssel enthält 30 Gattungen, 134 Arten, be used. -
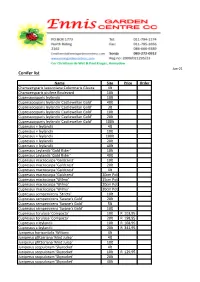
Conifer List
Jun-21 Conifer list Name Size Price Order Chamaecyparis lawsoniana Columnaris Glauca 4lt Chamaecyparis pisifere Boulevard 10lt Cupressocyparis leylandii 10lt Cupressocyparis leylandii 'Castlewellan Gold' 40lt Cupressocyparis leylandii 'Castlewellan Gold' 3lt Cupressocyparis leylandii 'Castlewellan Gold' 10lt Cupressocyparis leylandii 'Castlewellan Gold' 20lt Cupressocyparis leylandii 'Castlewellan Gold' 100lt Cupressus × leylandii 4lt Cupressus × leylandii 10lt Cupressus × leylandii 100lt Cupressus × leylandii 20lt Cupressus × leylandii 40lt Cupressus Leylandii 'Gold Rider' 10lt Cupressus Leylandii 'Gold Rider' 40lt Cupressus macrocarpa 'Goldcrest' 10lt Cupressus macrocarpa 'Goldcrest' 20lt Cupressus macrocarpa 'Goldcrest' 4lt Cupressus macrocarpa 'Goldcrest' 30cm Pot Cupressus macrocarpa 'Wilma' 15cm Pot Cupressus macrocarpa 'Wilma' 20cm Pot Cupressus macrocarpa 'Wilma' 30cm Pot Cupressus sempervirens 'Stricta' 10lt Cupressus sempervirens 'Swane's Gold' 20lt Cupressus sempervirens 'Swane's Gold' 5lt Cupressus sempervirens 'Swane's Gold' 10lt Cupressus torulosa 'Compacta' 10lt R 103,95 Cupressus torulosa 'Compacta' 20lt R 198,95 Cupressus x leylandii 10lt R 168,95 Cupressus x leylandii 20lt R 341,95 Juniperus horizontalis 'Wiltonii 4lt Juniperus pfitzeriana 'Mint Julep' 4lt Juniperus pfitzeriana 'Mint Julep' 10lt Juniperus scopulorum 'Skyrocket' 4lt Juniperus scopulorum 'Skyrocket' 10lt R 129,95 Juniperus scopulorum 'Skyrocket' 20lt Juniperus scopulorum 'Skyrocket' 40lt Juniperus scopulorum 'Skyrocket' 100lt Juniperus scopulorum 'Whitcha -

Nomenclature and Taxonomic Notes on Cupressus Gigantea Cheng & Fu
Bull. CCP 2 (1): 17-22. (6.2013) D. Mаеrki Nomenclature and Taxonomic Notes on Cupressus gigantea Cheng & Fu From material collected in Adelaide, Australia, labelled Cupressus torulosa D.Don var. majestica Carrière, sent to China by Mao & Liu for molecular analysis, Silba (2012) got the interesting result that this variety matches the species Cupressus gigantea described by Cheng & Fu in 1975. This taxon was first described by Carrière in his Traité Général des Conifères (1855) from a material earlier listed in the catalogue of the Knight & Perry nursery in United Kingdom (1850). If this taxon is considered as a variety of Cupressus torulosa, the correct name is therefore Cupressus torulosa var. majestica Carrière, and the combination Cupressus torulosa var. gigantea (Cheng & Fu) Farjon (2005) becomes superfluous. However, from morphological, physiological, molecular, ecological and geographical data, there is no justification to treat Cupressus gigantea as a variety of Cupressus torulosa D.Don. The two taxa are easily distinguished from one other. Morphology Leaves : Farjon discusses mainly shoot morphology, yet foliage in this genus is rarely easy to distinguish between the different species, with only very few exceptions such as Cupressus macnabiana A.Murray bis or Cupressus funebris Endlicher (adult foliage). Despite this, Cupressus gigantea can show white resin dots Fig. 1 : Mature cone of Cupressus gigantea, 17.2.2013. on adult leaves, while this has not been observed on C. torulosa. Foliage on saplings of Cupressus gigantea is typically glaucous, on C. torulosa green. Seed cones : they are easily distinguishable. C. gigantea cones display typical recurved umboes on the distal scales (see fig.