Technical Note 352
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

STORRE Veral Et Al. Tilapia Locomotor Activity .Pdf
CIRCADIAN RHYTHMS OF LOCOMOTOR ACTIVITY IN THE NILE TILAPIA OREOCHROMIS NILOTICUS Luisa María Veraa, Louise Cairnsa, Francisco Javier Sánchez-Vázquezb, Hervé Migauda* a Reproduction and Genetics Group, Institute of Aquaculture, University of Stirling. Stirling, UK. b Department of Physiology, Faculty of Biology, University of Murcia, 30100 Murcia, Spain. Running title: Circadian rhythms in tilapia *Corresponding author: Dr. Hervé Migaud Institute of Aquaculture, University of Stirling FK9 4LA, Stirling, UK Tel. 0044 1786 467886 Fax. 0044 1786 472133 E-mail: [email protected] 1 ABSTRACT The Nile tilapia behavioural rhythms were investigated to better characterize its circadian system. To do so, the locomotor activity patterns of both male and female tilapia reared under a 12: 12-h light-dark (LD) cycle were studied, as well as the existence of endogenous rhythmicity under free-running conditions (DD and 45-min LD pulses) in males. When exposed to an LD cycle, the daily pattern of activity differed between individuals: some fish were diurnal, some nocturnal and a few displayed an arrhythmic pattern. This variability would be typical of the plastic circadian system of fish and reproductive events clearly affected the behavioural rhythms of female tilapia, a mouthbrooder teleost species. Under DD, 50% (6 out of 12) of male fish showed circadian rhythms with an average period (tau) of 24.1 ± 0.2 h whereas under the 45- min LD pulses 58% (7 out of 12) of fish exhibited free-running activity rhythms and tau was 23.9 ± 0.5 h. However, interestingly in this case activity was always confined to the dark phase. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Sonora Sucker
scientific name common name Catostomus insignis Sonora sucker Bison code 010520 ______________________________________________________________ Official status Endemism ________________________ State AZ: threatened Colorado River Basin _______________________ Status/threats Dams, diversions, groundwater pumping and introduced species Distribution The species is widespread and abundant in the Gila and Bill Williams river drainages in Arizona and the Gila and San Francisco drainages in southwestern New Mexico. The species is widespread and abundant in the Verde and Gila headwaters. Habitat Streams and rivers from 300 to 3000 m in elevation, primarily in pool habitats. Pool habitats over sand gravel substrates. Life history and ecology Can attain a size of 0.8 m and a weight of greater than 2.0 kg. Used as food by early, primitive human populations. Food habits vary with availability. In one stream, Aravaipa Creek, it is principally a carnivore, whereas elsewhere in pool habitats diet consists of plant debris, mud, and algae. Observed to "suck" cottonwood seeds at surface as is common for the common carp. Young often feed in large schools at stream margins on micro-crustaceans, protozoans and other animal and plant groups. Breeding Similar to most slim-bodied suckers, the species spawns in smaller streams over gravel substrates. Males darken in color and often display extreme tuberculation. Males &(usually 2) flank a single, larger female. Gametes are emitted with considerable to extreme substrate agitation and fall into gravel interstices. Cleaning of gravels occurs much as reported for salmonid species. Key Habitat Components: pools with sand-gravel substrates for adults and shallow, low velocity riffles and backwaters for young Breeding season Protracted, from as early as January to February at low elevations to as late as July. -

Lessons from Genome Skimming of Arthropod-Preserving Ethanol Benjamin Linard, P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication Lessons from genome skimming of arthropod-preserving ethanol Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler To cite this version: Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler. Lessons from genome skimming of arthropod-preserving ethanol. Molecular Ecology Resources, Wiley/Blackwell, 2016, 16 (6), pp.1365-1377. 10.1111/1755-0998.12539. hal-01636888 HAL Id: hal-01636888 https://hal.archives-ouvertes.fr/hal-01636888 Submitted on 17 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Lessons from genome skimming of arthropod-preserving 2 ethanol 3 Linard B.*1,4, Arribas P.*1,2,5, Andújar C.1,2, Crampton-Platt A.1,3, Vogler A.P. 1,2 4 5 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 6 5BD, UK, 7 2 Department of Life Sciences, Imperial College London, Silwood Park Campus, Ascot 8 SL5 7PY, UK, 9 3 Department -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Gila Topminnow Revised Recovery Plan December 1998
GILA TOPMINNOW, Poeciliopsis occidentalis occidentalis, REVISED RECOVERY PLAN (Original Approval: March 15, 1984) Prepared by David A. Weedman Arizona Game and Fish Department Phoenix, Arizona for Region 2 U.S. Fish and Wildlife Service Albuquerque, New Mexico December 1998 Approved: Regional Director, U.S. Fish and Wildlife Service Date: Gila Topminnow Revised Recovery Plan December 1998 DISCLAIMER Recovery plans delineate reasonable actions required to recover and protect the species. The U.S. Fish and Wildlife Service (Service) prepares the plans, sometimes with the assistance of recovery teams, contractors, State and Federal Agencies, and others. Objectives are attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Time and costs provided for individual tasks are estimates only, and not to be taken as actual or budgeted expenditures. Recovery plans do not necessarily represent the views nor official positions or approval of any persons or agencies involved in the plan formulation, other than the Service. They represent the official position of the Service only after they have been signed by the Regional Director or Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. ii Gila Topminnow Revised Recovery Plan December 1998 ACKNOWLEDGMENTS Original preparation of the revised Gila topminnow Recovery Plan (1994) was done by Francisco J. Abarca 1, Brian E. Bagley, Dean A. Hendrickson 1 and Jeffrey R. Simms 1. That document was modified to this current version and the work conducted by those individuals is greatly appreciated and now acknowledged. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -

Laughing Waters" 40
Press, W. H., B. P. Flannery, S. A. Teukolsky, and W. T. Vetterling. 1986. Numerical recipes: C 1990 by S.E.L & Associates The art of scientific computing. Cambridge, MA: Cambridge University Press. Stalnaker, C. B. 1978. The IFG incremental methodology for physical instream habitat eval- uation. Washington, DC: U.S. Fish and Wildlife Service (FWS / OBS-78 /81). Stier, D. J., and J. H. Crance. 1985. Habitat suitability index models and instream flow suitability curves: American shad. Washington, DC: U.S. Fish and Wildlife Service (FWS/ OBS-82 /10). The Recreational Impact of Trihey, E. W. 1979. The IFG incremental methodology. Pages 24-44 in G. L. Smith, editor. Proceedings of the workshop in instream flow habitat criteria and modeling. Information Series Reducing the "Laughing Waters" 40. Fort Collins: Colorado State University, Colorado Water Resources Research Institute. Valdez, R. A., and B. C. Nilson. 1982. Radiotelemetry as a means of assessing movement and of Aravaipa Creek, Arizona habitat selection of humpback chub. Pages 29-39 in W. Geer, editor. Transactions of the Bonneville Chapter of the American Fisheries Society. Salt Lake City, UT: Bonneville Chapter of the American Fisheries Society. Steven D. Moore Valdez, R. A., P. B. Holden, T. B. Hardy, and R. J. Ryel. 1987. Habitat suitability index curves Mary E. Wilkosz* for endangered fishes of the Upper Colorado River Basin. Final report to US. Fish and Wildlife Service (Contract No. 14-16-0006-86-055), Denver, CO. Stanley K. Brickler Received: May 3, 1989 School of Renewable Natural Resources Accepted: June 25, 1989 University of Arizona Discussion open until August 1, 1990 Tucson, Arizona 85721 ABSTRACT: The paper describes analyses that were conducted to de- termine the importance of water as an attribute of the recreational setting at Aravaipa Canyon Wilderness, Arizona, and the influence that reduced flows in Aravaipa Creek would have on visitors' perceptions of water quantity and quality. -

Bugs R Al, No
ISSN 2230 – 7052 Newsletter of the $WIU4#NNInvertebrate Conservation & Information Network of South Asia (ICINSA) No. 22, MAY 2016 C. Sunil Kumar Photo: CONTENTS Pages Authenc report of Ceresium leucosccum White (Coleoptera: Cerambycidae: Callidiopini) from Pune and Satara in Maharashtra State --- Paripatyadar, S., S. Gaikwad and H.V. Ghate ... 2-3 First sighng of the Apefly Spalgis epeus epeus Westwood, 1851 (Lepidoptera: Lycaenidae: Milenae: Spalgini) from the Garhwal Himalaya --- Sanjay Sondhi ... 4-5 On a collecon of Odonata (Insecta) from Lonar (Crater) Lake and its environs, Buldhana district, Maharashtra, India --- Muhamed Jafer Palot ... 6-9 Occurrence of Phyllodes consobrina Westwood 1848 (Noctuidae: Lepidoptera) from Southern Western Ghats, India and a review of distribuonal records --- Prajith K.K., Anoop Das K.S., Muhamed Jafer Palot and Longying Wen ... 10-11 First Record of Gerosis bhagava Moore 1866 (Lepidoptera: Hesperiidae) from Bangladesh --- Ashis Kumar Daa ... 12 Present status on some common buerflies in Rahara area, West Bengal --- Wrick Chakraborty & Partha P. Biswas ... 13-17 Addions to the Buerfly fauna of Sundarbans Mangrove Forest, Bangladesh --- Ashis Kumar Daa ... 18 Study on buerfly (Papilionoidea) diversity of Bilaspur city --- Shubhada Rahalkar ... 19-23 Bio-ecology of Swallowtail (Lepidoptera:Papilionidae) Buerflies in Gautala Wildlife Sanctuary of Maharashtra India -- Shinde S.S. Nimbalkar R.K. and Muley S.P. ... 24-26 New report of midge gall (Diptera: Cecidomyiidae) on Ziziphus xylopyrus (Retz.) Willd. (Rhamnaceae) from Northern Western Ghats. Mandar N. Datar and R.M. Sharma ... 27 Rapid assessment of buerfly diversity in a ecotone adjoining Bannerghaa Naonal Park, South Bengaluru Alexander R. Avinash K. Phalke S. Manidip M. -

Native Fish Restoration in Redrock Canyon
U.S. Department of the Interior Bureau of Reclamation Final Environmental Assessment Phoenix Area Office NATIVE FISH RESTORATION IN REDROCK CANYON U.S. Department of Agriculture Forest Service Southwestern Region Coronado National Forest Santa Cruz County, Arizona June 2008 Bureau of Reclamation Finding of No Significant Impact U.S. Forest Service Finding of No Significant Impact Decision Notice INTRODUCTION In accordance with the National Environmental Policy Act of 1969 (Public Law 91-190, as amended), the Bureau of Reclamation (Reclamation), as the lead Federal agency, and the Forest Service, U.S. Fish and Wildlife Service (FWS), and Arizona Game and Fish Department (AGFD), as cooperating agencies, have issued the attached final environmental assessment (EA) to disclose the potential environmental impacts resulting from construction of a fish barrier, removal of nonnative fishes with the piscicide antimycin A and/or rotenone, and restoration of native fishes and amphibians in Redrock Canyon on the Coronado National Forest (CNF). The Proposed Action is intended to improve the recovery status of federally listed fish and amphibians (Gila chub, Gila topminnow, Chiricahua leopard frog, and Sonora tiger salamander) and maintain a healthy native fishery in Redrock Canyon consistent with the CNF Plan and ongoing Endangered Species Act (ESA), Section 7(a)(2), consultation between Reclamation and the FWS. BACKGROUND The Proposed Action is part of a larger program being implemented by Reclamation to construct a series of fish barriers within the Gila River Basin to prevent the invasion of nonnative fishes into high-priority streams occupied by imperiled native fishes. This program is mandated by a FWS biological opinion on impacts of Central Arizona Project (CAP) water transfers to the Gila River Basin (FWS 2008a). -

Herpetofauna and Aquatic Macro-Invertebrate Use of the Kino Environmental Restoration Project (KERP)
Herpetofauna and Aquatic Macro-invertebrate Use of the Kino Environmental Restoration Project (KERP) Tucson, Pima County, Arizona Prepared for Pima County Regional Flood Control District Prepared by EPG, Inc. JANUARY 2007 - Plma County Regional FLOOD CONTROL DISTRICT MEMORANDUM Water Resources Regional Flood Control District DATE: January 5,2007 TO: Distribution FROM: Julia Fonseca SUBJECT: Kino Ecosystem Restoration Project Report The Ed Pastor Environmental Restoration ProjectiKino Ecosystem Restoration Project (KERP) is becoming an extraordinary urban wildlife resource. As such, the Pima County Regional Flood Control District (PCRFCD) contracted with the Environmental Planning Group (EPG) to gather observations of reptiles, amphibians, and aquatic insects at KERP. Water quality was also examined. The purpose of the work was to provide baseline data on current wildlife use of the KERP site, and to assess water quality for post-project aquatic wildlife conditions. I additionally requested sampling of macroinvertebrates at Agua Caliente Park and Sweetwater Wetlands in hopes that the differences in aquatic wildlife among the three sites might provide insights into the different habitats offered by KERF'. The results One of the most important wildlife benefits that KERP provides is aquatic habitat without predatory bullfrogs and non- native fish. Most other constructed ponds and wetlands in Tucson, such as the Sweetwater Wetlands and Agua Caliente pond, are fuIl of non-native predators which devastate native fish, amphibians and aquatic reptiles. The KERP Wetlands may provide an opportunity for reestablishing declining native herpetofauna. Provided that non- native fish, bullfrogs or crayfish are not introduced, KERP appears to provide adequate habitat for Sonoran Mud Turtles (Kinosternon sonoriense), Lowland Leopard Frogs (Rana yavapaiensis), and Mexican Gartersnakes (Tharnnophis eques) and Southwestern Woodhouse Toad (Bufo woodhousii australis). -
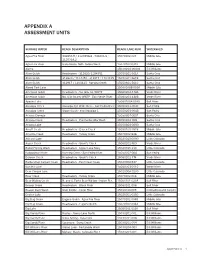
Appendix a Assessment Units
APPENDIX A ASSESSMENT UNITS SURFACE WATER REACH DESCRIPTION REACH/LAKE NUM WATERSHED Agua Fria River 341853.9 / 1120358.6 - 341804.8 / 15070102-023 Middle Gila 1120319.2 Agua Fria River State Route 169 - Yarber Wash 15070102-031B Middle Gila Alamo 15030204-0040A Bill Williams Alum Gulch Headwaters - 312820/1104351 15050301-561A Santa Cruz Alum Gulch 312820 / 1104351 - 312917 / 1104425 15050301-561B Santa Cruz Alum Gulch 312917 / 1104425 - Sonoita Creek 15050301-561C Santa Cruz Alvord Park Lake 15060106B-0050 Middle Gila American Gulch Headwaters - No. Gila Co. WWTP 15060203-448A Verde River American Gulch No. Gila County WWTP - East Verde River 15060203-448B Verde River Apache Lake 15060106A-0070 Salt River Aravaipa Creek Aravaipa Cyn Wilderness - San Pedro River 15050203-004C San Pedro Aravaipa Creek Stowe Gulch - end Aravaipa C 15050203-004B San Pedro Arivaca Cienega 15050304-0001 Santa Cruz Arivaca Creek Headwaters - Puertocito/Alta Wash 15050304-008 Santa Cruz Arivaca Lake 15050304-0080 Santa Cruz Arnett Creek Headwaters - Queen Creek 15050100-1818 Middle Gila Arrastra Creek Headwaters - Turkey Creek 15070102-848 Middle Gila Ashurst Lake 15020015-0090 Little Colorado Aspen Creek Headwaters - Granite Creek 15060202-769 Verde River Babbit Spring Wash Headwaters - Upper Lake Mary 15020015-210 Little Colorado Babocomari River Banning Creek - San Pedro River 15050202-004 San Pedro Bannon Creek Headwaters - Granite Creek 15060202-774 Verde River Barbershop Canyon Creek Headwaters - East Clear Creek 15020008-537 Little Colorado Bartlett Lake 15060203-0110 Verde River Bear Canyon Lake 15020008-0130 Little Colorado Bear Creek Headwaters - Turkey Creek 15070102-046 Middle Gila Bear Wallow Creek N. and S. Forks Bear Wallow - Indian Res.