Lessons from Bat Echolocationq
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Timeline of the Evolutionary History of Life
Timeline of the evolutionary history of life This timeline of the evolutionary history of life represents the current scientific theory Life timeline Ice Ages outlining the major events during the 0 — Primates Quater nary Flowers ←Earliest apes development of life on planet Earth. In P Birds h Mammals – Plants Dinosaurs biology, evolution is any change across Karo o a n ← Andean Tetrapoda successive generations in the heritable -50 0 — e Arthropods Molluscs r ←Cambrian explosion characteristics of biological populations. o ← Cryoge nian Ediacara biota – z ← Evolutionary processes give rise to diversity o Earliest animals ←Earliest plants at every level of biological organization, i Multicellular -1000 — c from kingdoms to species, and individual life ←Sexual reproduction organisms and molecules, such as DNA and – P proteins. The similarities between all present r -1500 — o day organisms indicate the presence of a t – e common ancestor from which all known r Eukaryotes o species, living and extinct, have diverged -2000 — z o through the process of evolution. More than i Huron ian – c 99 percent of all species, amounting to over ←Oxygen crisis [1] five billion species, that ever lived on -2500 — ←Atmospheric oxygen Earth are estimated to be extinct.[2][3] Estimates on the number of Earth's current – Photosynthesis Pong ola species range from 10 million to 14 -3000 — A million,[4] of which about 1.2 million have r c been documented and over 86 percent have – h [5] e not yet been described. However, a May a -3500 — n ←Earliest oxygen 2016 -
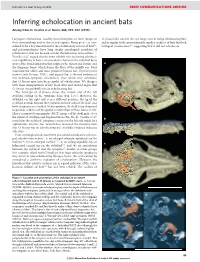
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

New European Bat Heroes, Not Villains Bats & Mosquitoes
SURPRISING HABITS OF A TEACHING KIDS THAT BATS ARE NEW EUROPEAN BAT BATS & M OSQUITOES HEROES , NOT VILLAINS WWW.BATCO N.ORG SPRING 2010 BBBAT CONSAAERVATIONTT INTERNASSTIONAL Volume 28, No. 1, spriNg 2010 P.O. Box 162603 , Austin, Texas 78716 BATS (512) 327-9721 • Fax (512) 327-9724 FEATURES Publications Staff Director of Publications: Robert Locke Photo Editor: Meera Banta 1 The Memo Graphic Artist: Jason Huerta Copyeditors: Angela England, Valerie Locke BATS welcomes queries from writers. Send your article proposal 2 Training for Research & Conservation with a brief outline and a description of any photos to the ad - in Latin America dress above or via email to: [email protected] . Members: Please send changes of address and all cor res - Workshops spur homegrown projects for bats pondence to the address above or via email to members@bat - con.org . Please include your label, if possible, and allow six by Christa Weise weeks for the change of address. Founder/President Emeritus: Dr. Merlin D. Tuttle Bats & Mosquitoes Executive Director: Nina Fascione 6 Board of Trustees: Testing conventional wisdom John D. Mitchell, Chair by Michael H. Reiskind and Matthew A. Wund Bert Grantges, Secretary Marshall T. Steves, Jr., Treasurer Anne-Louise Band; Eugenio Clariond Reyes; John 8 The Surprising Habits Hayes; C. Andrew Marcus; Bettina Mathis; Gary F. Mc - Cracken; Steven P. Quarles; Sandy Read; Walter C. Sedg - Of a New European Bat wick; Marc Weinberger. Advisory Trustees: Sharon R. Forsyth; Elizabeth Ames Alcathoe myotis face unique conservation challenges Jones; Travis Mathis; Wilhelmina Robertson; William by Radek K. Lu čan Scanlan, Jr.; Merlin D. -

Acoustic Identification of Mormoopid Bats: a Survey During the Evening Exodus
Journal of Mammalogy, 87(2):324–330, 2006 ACOUSTIC IDENTIFICATION OF MORMOOPID BATS: A SURVEY DURING THE EVENING EXODUS SILVIO MACI´AS,* EMANUEL C. MORA, AND ADIANEZ GARCI´A Department of Human and Animal Biology, Faculty of Biology, Havana University, CP 10 400, Ciudad de La Habana, Cuba (SM, ECM, AG) Department of Basic Formation, Faculty of Psychology, Havana University, Calle San Rafael No. 1168 entre Mazo´n y Basarrate, Centro Habana, Ciudad de La Habana, Cuba (SM) Echolocation calls emitted by the 4 species of Cuban mormoopid bats were compared to determine vocal signatures that enable identification of each species in the field during their evening exodus. Echolocation calls produced by Mormoops blainvilli are downward frequency-modulated (FM) signals in the range of 68.4– 52.5 kHz. Echolocation calls emitted by Pteronotus macleayii and P. quadridens have a similar design consisting of a short constant-frequency (CF) segment followed by a downward FM segment. The CF segment was at 70.0 kHz in calls from P. macleayii, and at 83.3 kHz in calls from P. quadridens. Echolocation calls from P. parnellii consist of a long CF segment, which is preceded by a short initial upward sweep and followed by a downward FM terminal sweep. The CF value of the 2nd harmonic was a good parameter for species identification. The features of the echolocation calls of each of the species were used to identify them during the evening exodus from 2 Cuban caves. Key words: echolocation, evening exodus, identification, mormoopid bats Effective monitoring of echolocation calls is vital in many families, such as Mormoopidae, should make it possible to use studies of the ecology and conservation of bats (Fenton 1997). -

Noctilio Leporinus (Chiroptera, Noctilionidae) from South America
AMERICAN MUSEUM NOVITATES Number 3798, 31 pp. April 4, 2014 Quaternary Bats from the Impossível-Ioiô Cave System (Chapada Diamantina, Brazil): Humeral Remains and the First Fossil Record of Noctilio leporinus (Chiroptera, Noctilionidae) from South America LEANDRO O. SALLES,1, 2 JOAQUÍN ARROYO-CABRALES,3 ANNE CARULINY DO MONTE LIMA,1 WAGNER LANZELOTTI,1 FERNANDO A. PERINI,4 PAÚL M. VELAZCO,2 AND NANCY B. SIMMONS2 ABSTRACT The partially submerged Impossível-Ioiô cave system located in the karst region of Cha- pada Diamantina in Bahia (Brazil) has recently been the target of extensive paleontological studies. Here we provide the first report of fossil bats from this cave system, in which we rec- ognize six species based on humeral remains: Furipterus horrens, Chrotopterus auritus, Mor- moops cf. megalophylla, Pteronotus gymnonotus, Pteronotus parnellii, and Noctilio leporinus. Morphology of the humerus of these taxa is described in a comparative framework to docu- ment taxonomic assessments and provide a basis for future studies of fossil bat faunas. The relevance of the new records reported here is evaluated at a broader continental scale, as well as in contrast with the recent bat fauna of the region. The record of Noctilio leporinus stands as the first fossil occurrence of this species on the South American continent. 1 Mastozoologia, Departamento de Vertebrados, Museu Nacional/UFRJ, Quinta da Boa Vista, s/n, Rio de Janeiro 20940-040, Brazil. 2 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History. 3 Laboratorio de Arqueozoología, Instituto Nacional de Antropología e Historia, Moneda # 16, Col. Centro México, D.F., México. 4 Departamento de Zoologia, Universidade Federal de Minas Gerais, Avenida Antônio Carlos 6627, Belo Horizonte 31270-901, Brazil. -

BATS of the Golfo Dulce Region, Costa Rica
MURCIÉLAGOS de la región del Golfo Dulce, Puntarenas, Costa Rica BATS of the Golfo Dulce Region, Costa Rica 1 Elène Haave-Audet1,2, Gloriana Chaverri3,4, Doris Audet2, Manuel Sánchez1, Andrew Whitworth1 1Osa Conservation, 2University of Alberta, 3Universidad de Costa Rica, 4Smithsonian Tropical Research Institute Photos: Doris Audet (DA), Joxerra Aihartza (JA), Gloriana Chaverri (GC), Sébastien Puechmaille (SP), Manuel Sánchez (MS). Map: Hellen Solís, Universidad de Costa Rica © Elène Haave-Audet [[email protected]] and other authors. Thanks to: Osa Conservation and the Bobolink Foundation. [fieldguides.fieldmuseum.org] [1209] version 1 11/2019 The Golfo Dulce region is comprised of old and secondary growth seasonally wet tropical forest. This guide includes representative species from all families encountered in the lowlands (< 400 masl), where ca. 75 species possibly occur. Species checklist for the region was compiled based on bat captures by the authors and from: Lista y distribución de murciélagos de Costa Rica. Rodríguez & Wilson (1999); The mammals of Central America and Southeast Mexico. Reid (2012). Taxonomy according to Simmons (2005). La región del Golfo Dulce está compuesta de bosque estacionalmente húmedo primario y secundario. Esta guía incluye especies representativas de las familias presentes en las tierras bajas de la región (< de 400 m.s.n.m), donde se puede encontrar c. 75 especies. La lista de especies fue preparada con base en capturas de los autores y desde: Lista y distribución de murciélagos de Costa Rica. Rodríguez -

Cranial Morphology and Bite Force in Bats
CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS A Thesis Presented to the faculty of the Department of Biological Sciences California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Biological Sciences by Jeffrey B. Changaris FALL 2017 © 2017 Jeffrey B. Changaris ALL RIGHTS RESERVED ii CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS A Thesis by Jeffrey B. Changaris Approved by: ____________________________________, Committee Chair Dr. Ronald M. Coleman ____________________________________, Second Reader Dr. Winston C. Lancaster ____________________________________, Third Reader Dr. Joseph Bahlman ___________________________ Date iii Student: Jeffrey B. Changaris I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the thesis. ____________________________, Graduate Coordinator _________________ Dr. James W. Baxter Date Department of Biological Sciences iv Abstract of CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS by Jeffrey B. Changaris Neotropical Ghost-Faced bats of the genus Mormoops (Order Chiroptera, Family Mormoopidae) have a radically upturned rostrum, or snout, while the other mormoopid genus, Pteronotus, has only a slight upturning of the rostrum. This type of difference in morphology between closely related taxa is likely to be the result of some sort of specialization. Observation of Mormoops blainvillei, the Antillean Ghost-Faced bat, reveals that they can open their mouths very wide relative to the size of their heads. Mormoopid bats are insectivorous with Mormoops blainvillei having a prey preference of large moths, but related species, such as Pteronotus quadridens, the Sooty Mustached bat, have a more varied diet with a large component of smaller hard-bodied beetles. -

Issue No. 22 June 2012 Feature Article Contents Study on The
Issue No. 22 June 2012 www.hkbiodiversity.net Feature Article Contents Study on the Distribution and Habitat Feature Article: Study on the Distribution and Habitat Characteristics of the Chinese Grassbird Characteristics of Chinese Grassbird (Graminicola striatus, 大草鶯) in Hong (Graminicola striatus, 大草鶯) Kong in Hong Kong page 1 Ivy W.Y. So1, Judy H.C. Wan1, W.H. Lee1, William W.W. Cheng2 Working Group Column: 1Bird Working Group Experimentation on the Use of 2Nature Conservation Division Bat Boxes in Hong Kong page 10 漁農自然護理署鳥類工作小組於2011年夏季進行一項有關大草 鶯(Graminicola striatus) 的生態研究,發現大草鶯於本港的分布與舊 An Estimation of the Current Population 有記錄相似,估計現時本港的大草鶯數目約有490隻,其生境於三 Size of Yellow-crested Cockatoo 月至九月主要為海拔200米以上、長度及密度高的草地,而芒屬則 (Cacatua sulphurea, 小葵花鳳頭鸚鵡) 是其生境中覆蓋率最高的植物。 in Hong Kong page 15 Background Rare Lizard Found: Bogadek’s The Chinese Grassbird (Graminicola striatus, 大草鶯) (Fig. 1) is a newly recognised species that has been split from the Indian Grassbird Burrowing Lizard (Dibamous bogadeki, (G. bengalensis; formerly known as the Rufous-rumped Grassbird). 香港雙足蜥) page 17 The split of the grassbirds, which was proposed in 2010 based on a morphological, vocal and genetic study (Leader et al., 2010), was recently accepted by the International Ornithologists’ Union in January 2012 (Gill & Donsker, 2012). Subscribing Hong Kong Biodiversity If you would like to have a copy, or Fig. 1. The Chinese Grassbird. if you know anyone who is interested in receiving a copy of this newsletter, please send the name, organisation, and email (soft copy) or postal addresses (hard copy) to the Article Editor. Chief Editor : Simon K.F. CHAN ([email protected]) Article Editor : Aidia S.W. -

Bat Rabies and Other Lyssavirus Infections
Prepared by the USGS National Wildlife Health Center Bat Rabies and Other Lyssavirus Infections Circular 1329 U.S. Department of the Interior U.S. Geological Survey Front cover photo (D.G. Constantine) A Townsend’s big-eared bat. Bat Rabies and Other Lyssavirus Infections By Denny G. Constantine Edited by David S. Blehert Circular 1329 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2009 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Constantine, D.G., 2009, Bat rabies and other lyssavirus infections: Reston, Va., U.S. Geological Survey Circular 1329, 68 p. Library of Congress Cataloging-in-Publication Data Constantine, Denny G., 1925– Bat rabies and other lyssavirus infections / by Denny G. Constantine. p. cm. - - (Geological circular ; 1329) ISBN 978–1–4113–2259–2 1. -

Mai Po Nature Reserve Management Plan: 2019-2024
Mai Po Nature Reserve Management Plan: 2019-2024 ©Anthony Sun June 2021 (Mid-term version) Prepared by WWF-Hong Kong Mai Po Nature Reserve Management Plan: 2019-2024 Page | 1 Table of Contents EXECUTIVE SUMMARY ................................................................................................................................................... 2 1. INTRODUCTION ..................................................................................................................................................... 7 1.1 Regional and Global Context ........................................................................................................................ 8 1.2 Local Biodiversity and Wise Use ................................................................................................................... 9 1.3 Geology and Geological History ................................................................................................................. 10 1.4 Hydrology ................................................................................................................................................... 10 1.5 Climate ....................................................................................................................................................... 10 1.6 Climate Change Impacts ............................................................................................................................. 11 1.7 Biodiversity ................................................................................................................................................