Deep-Water Sinkholes and Bioherms of South Florida and the Pourtalès Terrace — Habitat and Fauna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomy and Diversity of the Sponge Fauna from Walters Shoal, a Shallow Seamount in the Western Indian Ocean Region
Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region By Robyn Pauline Payne A thesis submitted in partial fulfilment of the requirements for the degree of Magister Scientiae in the Department of Biodiversity and Conservation Biology, University of the Western Cape. Supervisors: Dr Toufiek Samaai Prof. Mark J. Gibbons Dr Wayne K. Florence The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the NRF. December 2015 Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region Robyn Pauline Payne Keywords Indian Ocean Seamount Walters Shoal Sponges Taxonomy Systematics Diversity Biogeography ii Abstract Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region R. P. Payne MSc Thesis, Department of Biodiversity and Conservation Biology, University of the Western Cape. Seamounts are poorly understood ubiquitous undersea features, with less than 4% sampled for scientific purposes globally. Consequently, the fauna associated with seamounts in the Indian Ocean remains largely unknown, with less than 300 species recorded. One such feature within this region is Walters Shoal, a shallow seamount located on the South Madagascar Ridge, which is situated approximately 400 nautical miles south of Madagascar and 600 nautical miles east of South Africa. Even though it penetrates the euphotic zone (summit is 15 m below the sea surface) and is protected by the Southern Indian Ocean Deep- Sea Fishers Association, there is a paucity of biodiversity and oceanographic data. -

Proposal for a Revised Classification of the Demospongiae (Porifera) Christine Morrow1 and Paco Cárdenas2,3*
Morrow and Cárdenas Frontiers in Zoology (2015) 12:7 DOI 10.1186/s12983-015-0099-8 DEBATE Open Access Proposal for a revised classification of the Demospongiae (Porifera) Christine Morrow1 and Paco Cárdenas2,3* Abstract Background: Demospongiae is the largest sponge class including 81% of all living sponges with nearly 7,000 species worldwide. Systema Porifera (2002) was the result of a large international collaboration to update the Demospongiae higher taxa classification, essentially based on morphological data. Since then, an increasing number of molecular phylogenetic studies have considerably shaken this taxonomic framework, with numerous polyphyletic groups revealed or confirmed and new clades discovered. And yet, despite a few taxonomical changes, the overall framework of the Systema Porifera classification still stands and is used as it is by the scientific community. This has led to a widening phylogeny/classification gap which creates biases and inconsistencies for the many end-users of this classification and ultimately impedes our understanding of today’s marine ecosystems and evolutionary processes. In an attempt to bridge this phylogeny/classification gap, we propose to officially revise the higher taxa Demospongiae classification. Discussion: We propose a revision of the Demospongiae higher taxa classification, essentially based on molecular data of the last ten years. We recommend the use of three subclasses: Verongimorpha, Keratosa and Heteroscleromorpha. We retain seven (Agelasida, Chondrosiida, Dendroceratida, Dictyoceratida, Haplosclerida, Poecilosclerida, Verongiida) of the 13 orders from Systema Porifera. We recommend the abandonment of five order names (Hadromerida, Halichondrida, Halisarcida, lithistids, Verticillitida) and resurrect or upgrade six order names (Axinellida, Merliida, Spongillida, Sphaerocladina, Suberitida, Tetractinellida). Finally, we create seven new orders (Bubarida, Desmacellida, Polymastiida, Scopalinida, Clionaida, Tethyida, Trachycladida). -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

The Chemistry of Marine Sponges∗ 4 Sherif S
The Chemistry of Marine Sponges∗ 4 Sherif S. Ebada and Peter Proksch Contents 4.1 Introduction ................................................................................ 192 4.2 Alkaloids .................................................................................. 193 4.2.1 Manzamine Alkaloids ............................................................. 193 4.2.2 Bromopyrrole Alkaloids .......................................................... 196 4.2.3 Bromotyrosine Derivatives ....................................................... 208 4.3 Peptides .................................................................................... 217 4.4 Terpenes ................................................................................... 240 4.4.1 Sesterterpenes (C25)............................................................... 241 4.4.2 Triterpenes (C30).................................................................. 250 4.5 Concluding Remarks ...................................................................... 268 4.6 Study Questions ........................................................................... 269 References ....................................................................................... 270 Abstract Marine sponges continue to attract wide attention from marine natural product chemists and pharmacologists alike due to their remarkable diversity of bioac- tive compounds. Since the early days of marine natural products research in ∗The section on sponge-derived “terpenes” is from a review article published -

Zoogeography of Digenetic Trematodes from West African Marine Fishes1
192 PROCEEDINGS OF THE HELMINTHOLOGICAL SOCIETY Zoogeography of Digenetic Trematodes from West African Marine Fishes1 JACOB H. FISCHTHAL Department of Biological Sciences, State University of New York at Binghamton, Binghamton, New York 13901. ABSTRACT: Of the 107 species of trematodes found in West African (Mauritania to Gabon) marine fishes, 100 are allocated to 64 genera in 24 families while seven are immature didymozoids. Many of these genera are located in most of the world's seas with the exception of the polar seas; only five are en- demic to West Africa. The data for the 41 species known from West Africa and elsewhere, and those morphologically closest to the 55 endemic species, indicate that they are very widely distributed, particularly in the Western and North Atlantic, and Mediterranean. Historical and present- day events concerning physical and biological environmental factors and their effects on actual and po- tential hosts as well as on life cycle stages of the trematodes have resulted in the geographical distribution reported. The distribution of marine fishes has been emphasized to explain in part the trematode distribu- tion. Studies on the geographical distribution of (Gulf of Guinea from 5° S to 15° N) and digenetic trematodes of marine fishes in various warm temperate Mauritania have been pre- seas have been presented by Manter (1955, sented by Ekman (1953), Buchanan (1958), 1963, 1967), Szidat (1961), and Lebedev Longhurst (1962), and Ingham (1970). (1969), but West African waters were not included as sufficient data were not available Zoogeographical Distribution until more recently. The digenetic trematodes Of the 107 species of trematodes found in of West African marine fishes (mainly shore West African fishes, 100 are allocated to 64 and shelf inhabitants) have been reported by genera in 24 families while seven are immature Dollfus (1929, 1937a, b, 1946, 1951, 1960), didymozoids of unknown generic status (Ap- Dollfus and Capron (1958), Thomas (1959, pendix I). -

Order ZEIFORMES PARAZENIDAE Parazens P.C
click for previous page Zeiformes: Parazenidae 1203 Order ZEIFORMES PARAZENIDAE Parazens P.C. Heemstra, South African Institute for Aquatic Biodiversity, South Africa iagnostic characters: Small to moderate-sized (to 30 cm) oblong fishes, the head and body com- Dpressed; body depth slightly less than head length, contained 2.6 to 2.9 times in standard length; head naked, the bones thin and soft; opercular bones weakly serrate; mouth large, terminal, the upper jaw extremely protrusile; maxilla widely expanded posteriorly, and mostly exposed when mouth is closed; no supramaxilla; jaws with 1 or 2 rows of small, slender, conical teeth; vomer with a few short stout teeth;gill rakers (including rudiments) 2 on upper limb, 8 on lower limb.Eye diameter about 1/3 head length and slightly less than snout length.Branchiostegal rays 7.Dorsal fin divided, with 8 slender spines and 26 to 30 soft rays; anal fin with 1 minute spine and 30 to 32 soft rays; dorsal-, anal-, and pectoral-fin rays un- branched; caudal fin forked, with 11 principal rays and 9 branched rays; pectoral fin with 15 or 16 rays, shorter than eye diameter; pelvic fins with 1 unbranched and 5 or 6 branched soft rays, but no spine, fin origin posterior to a vertical at pectoral-fin base. Scales moderate in size, weakly ctenoid, and deciduous; 2 lateral lines originating on body at upper end of operculum and running posteriorly about 4 scale rows apart, gradually converging to form a single line on caudal peduncle. Caudal peduncle stout, the least depth about equal to its length and slightly less than eye diameter.Vertebrae 34.Colour: body reddish or silvery; large black blotch on anterior margin of dorsal fin. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Chapter 13. State of Deep-Sea Coral and Sponge Ecosystems of the U.S
State of Deep‐Sea Coral and Sponge Ecosystems of the Southeast United States Chapter 13 in The State of Deep‐Sea Coral and Sponge Ecosystems of the United States Report Recommended citation: Hourigan TF, Reed J, Pomponi S, Ross SW, David AW, Harter S (2017) State of Deep‐Sea Coral and Sponge Ecosystems of the Southeast United States. In: Hourigan TF, Etnoyer, PJ, Cairns, SD (eds.). The State of Deep‐Sea Coral and Sponge Ecosystems of the United States. NOAA Technical Memorandum NMFS‐OHC‐4, Silver Spring, MD. 60 p. Available online: http://deepseacoraldata.noaa.gov/library. STATE OF THE DEEP‐SEA CORAL AND SPONGE ECOSYSTEMS OF THE SOUTHEAST UNITED STATES Squat lobster perched on Lophelia pertusa colonies with a sponge in the background. Courtesy of NOAA/ USGS. 408 STATE OF THE DEEP‐SEA CORAL AND SPONGE ECOSYSTEMS OF THE SOUTHEAST UNITED STATES STATE OF THE DEEP- SEA CORAL AND Thomas F. Hourigan1*, SPONGE ECOSYSTEMS John Reed2, OF THE SOUTHEAST Shirley Pomponi2, UNITED STATES Steve W. Ross3, Andrew W. David4, and I. Introduction Stacey Harter4 The Southeast U.S. region stretches from the Straits of Florida north to Cape Hatteras, North Carolina, and encompasses the 1 NOAA Deep Sea Coral Southeast U.S. Continental Shelf large marine ecosystem (LME; Research and Technology Carolinian ecoregion) and associated deeper waters of the Blake Program, Office of Habitat Plateau, as well as a small portion of the Caribbean LME off the Conservation, Silver Florida Keys (eastern portion of the Floridian ecoregion). Within Spring, MD * Corresponding Author: U.S. waters, deep‐sea stony coral reefs reach their greatest [email protected] abundance and development in this region (Ross and Nizinski 2007). -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -
Field Identification Guide to the Living Marine Resources in Kenya
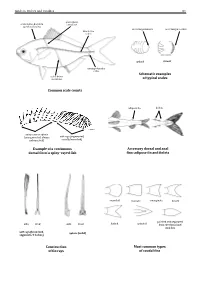
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Evolution and Ecology in Widespread Acoustic Signaling Behavior Across Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.14.296335; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Evolution and Ecology in Widespread Acoustic Signaling Behavior Across Fishes 2 Aaron N. Rice1*, Stacy C. Farina2, Andrea J. Makowski3, Ingrid M. Kaatz4, Philip S. Lobel5, 3 William E. Bemis6, Andrew H. Bass3* 4 5 1. Center for Conservation Bioacoustics, Cornell Lab of Ornithology, Cornell University, 159 6 Sapsucker Woods Road, Ithaca, NY, USA 7 2. Department of Biology, Howard University, 415 College St NW, Washington, DC, USA 8 3. Department of Neurobiology and Behavior, Cornell University, 215 Tower Road, Ithaca, NY 9 USA 10 4. Stamford, CT, USA 11 5. Department of Biology, Boston University, 5 Cummington Street, Boston, MA, USA 12 6. Department of Ecology and Evolutionary Biology and Cornell University Museum of 13 Vertebrates, Cornell University, 215 Tower Road, Ithaca, NY, USA 14 15 ORCID Numbers: 16 ANR: 0000-0002-8598-9705 17 SCF: 0000-0003-2479-1268 18 WEB: 0000-0002-5669-2793 19 AHB: 0000-0002-0182-6715 20 21 *Authors for Correspondence 22 ANR: [email protected]; AHB: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.14.296335; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.