Evaluation of Suprascapular Nerve Neurotization After Nerve Grafting Or Transfer in the Treatment of Brachial Plexus Traction Lesions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
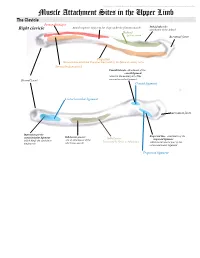
Muscle Attachment Sites in the Upper Limb
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Muscle Attachment Sites in the Upper Limb The Clavicle Pectoralis major Smooth superior surface of the shaft, under the platysma muscle Deltoid tubercle: Right clavicle attachment of the deltoid Deltoid Axillary nerve Acromial facet Trapezius Sternocleidomastoid and Trapezius innervated by the Spinal Accessory nerve Sternocleidomastoid Conoid tubercle, attachment of the conoid ligament which is the medial part of the Sternal facet coracoclavicular ligament Conoid ligament Costoclavicular ligament Acromial facet Impression for the Trapezoid line, attachment of the costoclavicular ligament Subclavian groove: Subclavius trapezoid ligament which binds the clavicle to site of attachment of the Innervated by Nerve to Subclavius which is the lateral part of the the first rib subclavius muscle coracoclavicular ligament Trapezoid ligament This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. The Scapula Trapezius Right scapula: posterior Levator scapulae Supraspinatus Deltoid Deltoid and Teres Minor are innervated by the Axillary nerve Rhomboid minor Levator Scapulae, Rhomboid minor and Rhomboid Major are innervated by the Dorsal Scapular Nerve Supraspinatus and Infraspinatus innervated by the Suprascapular nerve Infraspinatus Long head of triceps Rhomboid major Teres Minor Teres Major Teres Major -
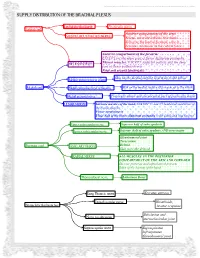
Supply Distribution of the Brachial Plexus
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. SUPPLY DISTRIBUTION OF THE BRACHIAL PLEXUS Lateral pectoral nerve Pectoralis major Lateral cord Anterior compartment of the arm: MUSCULOCUTANEOUS NERVE Biceps, coracobrachialis, brachialis Skin over the lateral forearm, once it becomes cutaneous in the cubital fossa Anterior compartment of the forearm: EXCEPT for the ulnar part of flexor digitorum profundis MEDIAN NERVE Thenar muscles: EXCEPT adductor pollicis and the deep part of flexor pollicis brevis First and second lumbricals Skin on the medial surface of arm up to the elbow MedialLateral cutaneous cord: nerve of arm lateral pectoral nerve Medial cord Skin on the medial surface of forearm up to the elbow Medial cutaneous MUSCULOCUTANEOUS nerve of forearm NERVE lateral root of MEDIAN NERVE Medial pectoral nerve Pectoralis minor and sternocostal part of pectoralis major o Medial cord forms ULNAR NERVE Intrinsic muscles of the hand, EXCEPT 1st and 2nd lumbricals and three of the thenar muscles Flexor carpi ulnaris Ulnar half of the flexor digitorum profundis to the pinky and ring fingers Upper subscapular nerve Superior half of subscapularis medial root of MEDIAN NERVE Lower subscapular nerve Inferior half of subscapularis AND teres major medial pectoral nerve medial cutaneous nerveGlenohumeral of arm joint Teres minor medial cutaneous nerve of forearm Posterior cord AXILLARY NERVE Deltoid ULNAR NERVE Skin over the deltoid RADIAL NERVE ALL MUSCLES IN THE POSTERIOR COMPARTMENT OF THE ARM AND FOREARM Ski over posterior and inferolateral forearm Some of the dorsum of the hand o o Thoracodorsal nerve Latissimus Dorsi o o Posterior cord forms: Long Thoracic nerve Serratus anterior Dorsal scapular nerve Rhomboids; Supraclavicular branches levator scapulae Subclavius and Nerve to subclavius sternoclavicular joint Suprascapular nerve Supraspinatus Infraspinatus Glenohumeral joint. -

Pectoral Region and Axilla Doctors Notes Notes/Extra Explanation Editing File Objectives
Color Code Important Pectoral Region and Axilla Doctors Notes Notes/Extra explanation Editing File Objectives By the end of the lecture the students should be able to : Identify and describe the muscles of the pectoral region. I. Pectoralis major. II. Pectoralis minor. III. Subclavius. IV. Serratus anterior. Describe and demonstrate the boundaries and contents of the axilla. Describe the formation of the brachial plexus and its branches. The movements of the upper limb Note: differentiate between the different regions Flexion & extension of Flexion & extension of Flexion & extension of wrist = hand elbow = forearm shoulder = arm = humerus I. Pectoralis Major Origin 2 heads Clavicular head: From Medial ½ of the front of the clavicle. Sternocostal head: From; Sternum. Upper 6 costal cartilages. Aponeurosis of the external oblique muscle. Insertion Lateral lip of bicipital groove (humerus)* Costal cartilage (hyaline Nerve Supply Medial & lateral pectoral nerves. cartilage that connects the ribs to the sternum) Action Adduction and medial rotation of the arm. Recall what we took in foundation: Only the clavicular head helps in flexion of arm Muscles are attached to bones / (shoulder). ligaments / cartilage by 1) tendons * 3 muscles are attached at the bicipital groove: 2) aponeurosis Latissimus dorsi, pectoral major, teres major 3) raphe Extra Extra picture for understanding II. Pectoralis Minor Origin From 3rd ,4th, & 5th ribs close to their costal cartilages. Insertion Coracoid process (scapula)* 3 Nerve Supply Medial pectoral nerve. 4 Action 1. Depression of the shoulder. 5 2. Draw the ribs upward and outwards during deep inspiration. *Don’t confuse the coracoid process on the scapula with the coronoid process on the ulna Extra III. -

A Comprehensive Review of Anatomy and Regional Anesthesia Techniques of Clavicle Surgeries
vv ISSN: 2641-3116 DOI: https://dx.doi.org/10.17352/ojor CLINICAL GROUP Received: 31 March, 2021 Research Article Accepted: 07 April, 2021 Published: 10 April, 2021 *Corresponding author: Dr. Kartik Sonawane, Uncovering secrets of the Junior Consultant, Department of Anesthesiol- ogy, Ganga Medical Centre & Hospitals, Pvt. Ltd. Coimbatore, Tamil Nadu, India, E-mail: beauty bone: A comprehensive Keywords: Clavicle fractures; Floating shoulder sur- gery; Clavicle surgery; Clavicle anesthesia; Procedure review of anatomy and specific anesthesia; Clavicular block regional anesthesia techniques https://www.peertechzpublications.com of clavicle surgeries Kartik Sonawane1*, Hrudini Dixit2, J.Balavenkatasubramanian3 and Palanichamy Gurumoorthi4 1Junior Consultant, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India 2Fellow in Regional Anesthesia, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India 3Senior Consultant, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India 4Consultant, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India Abstract The clavicle is the most frequently fractured bone in humans. General anesthesia with or without Regional Anesthesia (RA) is most frequently used for clavicle surgeries due to its complex innervation. Many RA techniques, alone or in combination, have been used for clavicle surgeries. These include interscalene block, cervical plexus (superficial and deep) blocks, SCUT (supraclavicular nerve + selective upper trunk) block, and pectoral nerve blocks (PEC I and PEC II). The clavipectoral fascial plane block is also a safe and simple option and replaces most other RA techniques due to its lack of side effects like phrenic nerve palsy or motor block of the upper limb. -

Electrodiagnosis of Brachial Plexopathies and Proximal Upper Extremity Neuropathies
Electrodiagnosis of Brachial Plexopathies and Proximal Upper Extremity Neuropathies Zachary Simmons, MD* KEYWORDS Brachial plexus Brachial plexopathy Axillary nerve Musculocutaneous nerve Suprascapular nerve Nerve conduction studies Electromyography KEY POINTS The brachial plexus provides all motor and sensory innervation of the upper extremity. The plexus is usually derived from the C5 through T1 anterior primary rami, which divide in various ways to form the upper, middle, and lower trunks; the lateral, posterior, and medial cords; and multiple terminal branches. Traction is the most common cause of brachial plexopathy, although compression, lacer- ations, ischemia, neoplasms, radiation, thoracic outlet syndrome, and neuralgic amyotro- phy may all produce brachial plexus lesions. Upper extremity mononeuropathies affecting the musculocutaneous, axillary, and supra- scapular motor nerves and the medial and lateral antebrachial cutaneous sensory nerves often occur in the context of more widespread brachial plexus damage, often from trauma or neuralgic amyotrophy but may occur in isolation. Extensive electrodiagnostic testing often is needed to properly localize lesions of the brachial plexus, frequently requiring testing of sensory nerves, which are not commonly used in the assessment of other types of lesions. INTRODUCTION Few anatomic structures are as daunting to medical students, residents, and prac- ticing physicians as the brachial plexus. Yet, detailed understanding of brachial plexus anatomy is central to electrodiagnosis because of the plexus’ role in supplying all motor and sensory innervation of the upper extremity and shoulder girdle. There also are several proximal upper extremity nerves, derived from the brachial plexus, Conflicts of Interest: None. Neuromuscular Program and ALS Center, Penn State Hershey Medical Center, Penn State College of Medicine, PA, USA * Department of Neurology, Penn State Hershey Medical Center, EC 037 30 Hope Drive, PO Box 859, Hershey, PA 17033. -

Tricks and Techniques to Maximize Success with Nerve Transfers
IC12-L: Tricks and Techniques to Maximize Success with Nerve Transfers Moderator(s): Susan E. Mackinnon, MD Faculty: Christine B. Novak, PT, PhD and J. Megan Patterson, MD Session Handouts Friday, October 02, 2020 75TH VIRTUAL ANNUAL MEETING OF THE ASSH OCTOBER 1-3, 2020 822 West Washington Blvd Chicago, IL 60607 Phone: (312) 880-1900 Web: www.assh.org Email: [email protected] All property rights in the material presented, including common-law copyright, are expressly reserved to the speaker or the ASSH. No statement or presentation made is to be regarded as dedicated to the public domain. IC12 – Tricks and Techniques to Maximize Success with Nerve Transfers American Society for Surgery of the Hand Annual Meeting, October 2, 2020 Susan E. Mackinnon, MD, St. Louis, Missouri J. Megan M. Patterson, Chapel Hill, North Carolina Christine B. Novak, PT, PhD, Toronto, Ontario Andrew Yee, BS, St. Louis, Missouri Overview: Following complex nerve injury, motor and sensory recovery can be less than optimal. For high median, ulnar and radial nerve injuries, nerve transfers can provide a distal source of motor and/or sensory innervation closer to the target end organ allowing for faster recovery and improved outcome. This course will focus on techniques to maximize success with upper extremity motor and sensory nerve transfers. Classification of Nerve Injury: Degree of Injury Tinel’s Sign Recovery Rate of Surgical Procedure Present Recovery I Neurapraxia No Complete Up to 12 weeks None II Axonotmesis Yes Complete 1” per month None III Yes Varies * -

SŁOWNIK ANATOMICZNY (ANGIELSKO–Łacinsłownik Anatomiczny (Angielsko-Łacińsko-Polski)´ SKO–POLSKI)
ANATOMY WORDS (ENGLISH–LATIN–POLISH) SŁOWNIK ANATOMICZNY (ANGIELSKO–ŁACINSłownik anatomiczny (angielsko-łacińsko-polski)´ SKO–POLSKI) English – Je˛zyk angielski Latin – Łacina Polish – Je˛zyk polski Arteries – Te˛tnice accessory obturator artery arteria obturatoria accessoria tętnica zasłonowa dodatkowa acetabular branch ramus acetabularis gałąź panewkowa anterior basal segmental artery arteria segmentalis basalis anterior pulmonis tętnica segmentowa podstawna przednia (dextri et sinistri) płuca (prawego i lewego) anterior cecal artery arteria caecalis anterior tętnica kątnicza przednia anterior cerebral artery arteria cerebri anterior tętnica przednia mózgu anterior choroidal artery arteria choroidea anterior tętnica naczyniówkowa przednia anterior ciliary arteries arteriae ciliares anteriores tętnice rzęskowe przednie anterior circumflex humeral artery arteria circumflexa humeri anterior tętnica okalająca ramię przednia anterior communicating artery arteria communicans anterior tętnica łącząca przednia anterior conjunctival artery arteria conjunctivalis anterior tętnica spojówkowa przednia anterior ethmoidal artery arteria ethmoidalis anterior tętnica sitowa przednia anterior inferior cerebellar artery arteria anterior inferior cerebelli tętnica dolna przednia móżdżku anterior interosseous artery arteria interossea anterior tętnica międzykostna przednia anterior labial branches of deep external rami labiales anteriores arteriae pudendae gałęzie wargowe przednie tętnicy sromowej pudendal artery externae profundae zewnętrznej głębokiej -

Langer's Axillary Arch (Axillopectoral Muscle): a Variation of Latissimus
eISSN 1308-4038 International Journal of Anatomical Variations (2010) 3: 91–92 Case Report Langer’s axillary arch (axillopectoral muscle): a variation of latissimus dorsi muscle Published online June 30th, 2010 © http://www.ijav.org Sinan BAKIRCI ABSTRACT Ilker Mustafa KAFA Langer’s axillary arch (axillopectoral muscle) is a variant muscular structure of the axilla which was described Murat UYSAL under various names as Langer’s muscle, axillary arch or muscular axillary arch by different authors. During Erdogan SENDEMIR routine dissections, we found a muscular slip on the right axillary fossa that originated from latissimus dorsi muscle and attached to the deep surface of the tendon of pectoralis major muscle, and described it as Langer’s axillary arch. Arterial, venous and nervous structures passed under this muscular slip which constitutes an arch in the axillary fossa. Although axillary arch is not very rare, it is generally neglected and not explored or described well. It has immense clinical and morphologic importance for surgical operations performed on axillary region; thus, surgeons should well be aware of its possible existence. © IJAV. 2010; 3: 91–92. Department of Anatomy, Faculty of Medicine, Uludag University, Bursa, TURKEY. Ilker Mustafa Kafa, MD Department of Anatomy Uludag University Faculty of Medicine Gorukle, 16059, Bursa, TURKEY. +90 (224) 295 23 16 [email protected] Received November 20th, 2009; accepted June 21st, 2010 Key words [axillary arch] [Langer’s muscle] [latissimus dorsi muscle] [axillopectoral muscle] [axillary fossa] Introduction os ilium. The axillary arch is a variant muscular slip of Best-known variant structure of the axillary components this muscle and is about 7 to 10 cm in length, splits from of men is a muscular or fibro-muscular slip extending the upper edge of the latissimus dorsi and crosses the from the latissimus dorsi muscle to the tendons, muscles or axilla in front of the axillary vessels and nerves [4]. -

Anatomical Study of Pectoral Nerves and Its Implications in Surgery a Natomy S Ection
DOI: 10.7860/JCDR/2014/8631.4545 Original Article ection Anatomical Study of Pectoral Nerves and S its Implications in Surgery natomy A PRAKASH KG1, SANIYA K2 ABSTRACT ramify within the muscle supplying it, finally runs along the Introduction: This anatomical study of the pectoral nerves lateral aspect (lower border) of the pectoralis minor muscle and their innervation is to provide detail informations on the to supply the lower portion or distal segment of the pectoralis pectoral nerves and their variations in their course, to guide major muscle. Similarly, the lateral pectoral nerve runs along the cosmetic and plastic surgeons for their easy intra operative the upper border (medial aspect) of the pectoralis minor muscle localization and to improve the understanding of the pectoral (98%) and then runs under surface of the pectoralis major muscle innervation, which is very much required during breast muscle along with the pectoral branch of thoracoacromial reconstruction after modified radical mastectomy (MRM) in artery, supplying the upper portion or most of the proximal 2/3rd breast cancer; axillary dissection; removal of the pectoralis of the pectoralis major muscle. Therefore, when the pectoralis minor muscle, and in harvesting the pectoralis major for minor muscle is removed in a modified radical mastectomy or myocutaneous head and neck island flap surgeries. during dissection between the two muscles, there is partial denervation of the pectoralis major muscle with partial atrophy Materials and Methods: A total of 50 pectoral region specimens and decrease in muscle mass. If the lateral pectoral nerve also (both right and left sided) from 25 embalmed adult human injured along with the medial pectoral nerve, it can result in total cadavers (20 female & 05 male) were studied by dissection denervation of the pectoralis major muscle with severe atrophy method. -

Abrant Origion and Distribution of Pectoral Nerves with Prominent Ansa Pectoralis and Absent Lateral Pectoral Nerve Chernet Bahru Tessema*
Case Report Abrant origion and distribution of pectoral nerves with prominent ansa pectoralis and absent lateral pectoral nerve Chernet Bahru Tessema* Tessema CB. Abrant origion and distribution of pectoral nerves with that distributed to the two pectoralis muscles. Two of the ansa pectoralis prominent ansa pectoralis and absent lateral pectoral nerve. Int J Anat branchesentered pectoralis major, one ended on pectoralis minor and one Var. 2020;13(2):1-3. entered both muscles. No lateral pectoral nerve from the lateral cord was observed. ABSTRACT Though the presence of multiple branches like in this case would make During the dissection of the left axilla of an 80-year-old male cadaver three approach to the pectoral region difficult without damage to these nerves, it anterior and one medial pectoral nerves were incidentally detected. All the could safeguard the normal muscle function in situation of isolated nerve three anterior pectoral nerve arose from the anterior division of the middle injuries. trunk of the brachial plexus. The upper two anterior pectoral nerves entered pectoralis major while the most inferior joined the medial pectoral nerve to Key Words: Brachial plexus; Anterior pectoral nerves; Medial pectoral nerve; Ansa form a prominent ansa pectoralis. The ansa pectorals then gave four branches pectoralis. INTRODUCTION entered the clavicular part of pectoralis major and the middle terminated in the most superior fibers of the sternocostal part of pectoralis major closer to he pectoralis muscles are innervated by the medial and lateral pectoral its clavicular part (Figures 1-3). The inferior anterior pectoral nerve joined Tnerves, which are branches of the respective cords of the brachial the medial pectoral nerve to form a loop, the ansa pectoralis (Figures 1 and plexus. -

Innervation of the Clavicular Part of the Deltoid Muscle by the Lateral Pectoral Nerve
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Innervation of the clavicular part of the deltoid muscle by the lateral pectoral nerve Larionov, Alexey ; Yotovski, Peter ; Link, Karl ; Filgueira, Luis Abstract: INTRODUCTION: The innervation pattern of the clavicular head of the deltoid muscle and its corresponding topography were investigated via cadaveric dissection in the present study, focusing on the lateral pectoral nerve. MATERIALS AND METHODS: Fifty-eight upper extremities were dissected and the nerve supplies to the deltoid muscle and the variability of the lateral pectoral and axillary nerves, including their topographical patterns, were noted. RESULTS: The clavicular portion of the deltoid muscle received a deltoid branch from the lateral pectoral nerve in 86.2% of cases. Two topographical patterns of the lateral pectoral nerve were observed, depending on the branching level from the brachial plexus: a proximal variant, where the nerve entered the pectoral region undern the clavicle, and a distal variant, where the nerve entered the pectoral region from the axillary fossa around the caudal border of the pectoralis minor. These dissection findings were supported by histological confirmation of peripheral nerve tissue entering the clavicular part of the deltoid muscle. CONCLUSION: The topographical variations of the lateral pectoral nerve are relevant for orthopedic and trauma surgeons and neurologists. These new data could revise the interpretation of deltoid muscle atrophy and of thoracic outlet and pectoralis minor compression syndromes. They could also explain the residual anteversion function of the arm after axillary nerve injury and deficiency, which is often thought to be related to biceps brachii muscle function. -

Nerve-Transfers-Lee
Review Article Nerve Transfers for the Upper Extremity: New Horizons in Nerve Reconstruction Abstract Steve K. Lee, MD Nerve transfers are key components of the surgeon’s Scott W. Wolfe, MD armamentarium in brachial plexus and complex nerve reconstruction. Advantages of nerve transfers are that nerve regeneration distances are shortened, pure motor or sensory nerve fascicles can be selected as donors, and nerve grafts are generally not required. Similar to the principle of tendon transfers, expendable donor nerves are transferred to denervated nerves with the goal of functional recovery. Transfers may be subdivided into intraplexal, extraplexal, and distal types; each has a unique role in From the Hospital for Special the reconstructive process. A thorough diagnostic workup and Surgery and Weill Cornell Medical intraoperative assessment help guide the surgeon in their use. College, New York, NY. Nerve transfers have made a positive impact on the outcomes of Dr. Lee or an immediate family nerve surgery and are essential tools in complex nerve member has received royalties from, is a member of a speakers’ bureau reconstruction. or has made paid presentations on behalf of, and serves as a paid consultant to or is an employee of Arthrex; serves as an unpaid lthough not a new concept, neurotization should be reserved to consultant to Synthes; has received Anerve transfers have become an describe the direct implantation of a research or institutional support from increasingly important technique in divided donor nerve into muscle, Arthrex, DePuy Mitek, Integra LifeSciences, Medartis, Axogen, and the strategic algorithm for nerve re- which has shown promise in an ani- 1,2 Checkpoint; and serves as a board construction.