The Morphology and Evolution of the Primate Brachial Plexus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ischaemic Lumbosacral Plexopathy in Acute Vascular Compromise:Case Report
Parapkgia 29 (1991) 70-75 © 1991 International Medical Soci<ty of Paraplegia Paraplegia L-_________________________________________________ � Ischaemic Lumbosacral Plexopathy in Acute Vascular Compromise: Case Report D.X. Cifu, MD, K.D. Irani, MD Department of Physical Medicine, Baylor College of Medicine, Houston, Texas, USA. Summary Anterior spinal artery syndrome (ASAS) is a well reported cause of spinal cord injury (SCI) following thoracoabdominal aortic surgery. The resultant deficits are often incom plete, typically attributed to the variable nature of the vascular distribution. Our Physi cal Medicine and Rehabilitation (PM and Rehabilitation) service was consulted about a 36-year-old patient with generalised deconditioning, 3 months after a stab wound to the left ventricle. Physical examination revealed marked lower extremity weakness, hypo tonia, hyporeflexia, and a functioning bowel and bladder. Further questioning disclosed lower extremity dysesthesias. Nerve conduction studies showed slowed velocities, pro longed distal latencies and decreased amplitudes of all lower extremity nerves. Electro myography revealed denervation of all proximal and distal lower extremity musculature, with normal paraspinalis. Upper extremity studies were normal. Recently, 3 cases of ischaemic lumbosacral plexopathy, mimicking an incomplete SCI, have been reported. This distinction is particularly difficult in the poly trauma patient with multiple musculo skeletal injuries or prolonged recuperation time, in addition to a vascular insult, as in this patient. The involved anatomical considerations will be discussed. A review of the elec trodiagnostic data from 30 patients, with lower extremity weakness following acute ischaemia, revealed a 20% incidence of spinal cord compromise, but no evidence of a plexopathy. Key words: Ischaemia; Lumbosacral plexopathy; Electromyography. Recent advances in cardiovascular and trauma surgery have led to increased survi val of patients following cardiac and great vessel trauma or insult. -

Study of Brachial Plexus with Regards to Its Formation, Branching Pattern and Variations and Possible Clinical Implications of Those Variations
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 4 Ver. VII (Apr. 2016), PP 23-28 www.iosrjournals.org Study of Brachial Plexus With Regards To Its Formation, Branching Pattern and Variations and Possible Clinical Implications of Those Variations. Dr. Shambhu Prasad1, Dr. Pankaj K Patel2 1 (Assistant Professor , Department of Anatomy , NMCH , Sasaram , India ) 2 (Assistant Professor , Department of Pathology , NMCH , Sasaram , India ) Abstract: Aim of the study: to search variations in formation and branching pattern of brachial plexus and correlate them with possible clinical and surgical implications. Materials and Methods: 25human bodies were dissected for this study. Dissection was started in posterior triangle of neck and extended to distal part of upper limb passing through axilla . Photographs were taken and data was tabulated and analyzed . Observations: All 50 plexuses had origin from C5 – T1 . Dorsal scapular nerve was absent in 2 plexuses. Long thoracic nerve was made of C5,C6 fibers in one and of C6,C7 fibers in another . Lower trunk was abnormal in 2 plexuses both of same body, their was no contribution from T1 fibers to posterior cord in one of these plexus. One plexus had double lateral pectoral nerve , communication between lateral and medial pectoral nerves , lateral pectoral and medial root of median nerve also between musculocutaneous and lateral root of median nerve . 2 more plexuses had communication between lateral and medial pectoral nerves. One plexus showed 2 branches coming from posterior division of upper trunk itself before formation of posterior cord. -

Check List the Journal Of
12 3 1906 the journal of biodiversity data 19 June 2016 Check List NOTES ON GEOGRAPHIC DISTRIBUTION Check List 12(3): 1906, 19 June 2016 doi: http://dx.doi.org/10.15560/12.3.1906 ISSN 1809-127X © 2016 Check List and Authors A new population of the endangered Brachyteles arachnoides (É. Geoffroy, 1806) (Primates: Atelidae) in the state of Paraná, southern Brazil Bianca Ingberman1*, Nicholas Kaminski2, Roberto Fusco-Costa1 and Emygdio L.A. Monteiro-Filho1, 3 1 Instituto de Pesquisas Cananéia (IPeC), Wildlife Research Department, Rua Tristão Lobo 199, Centro, CEP 11990-000, Cananéia, SP, Brazil 2 Fundação Neotrópica do Brasil, Rua Dois de Outubro, 165, Bairro Recreio, CEP 79290-000, Bonito, MS, Brazil 3 Universidade Federal do Paraná, Centro Politécnico, Setor de Ciências Biológicas, Departamento de Zoologia, Laboratório de Biologia e Ecologia de Vertebrados, Caixa Postal 19020, Jardim das Américas, CEP 81531-990, Curitiba, PR, Brazil * Corresponding author. E-mail: [email protected] Abstract: The endangered southern muriqui or mono these states, but the distribution is much more restrict- [Brachyteles arachnoides (É. Geoffroy, 1806)], is a primate ed, fragmented and poorly known. Its presence in the endemic to the Atlantic Forest of Brazil. One known state of Paraná was suggested by Krieg (1939 apud Hill extant population is found at the southern limit of its 1962: 357), who stated that “… the range extends south- distribution, in the state of Paraná, where it is regionally wards beyond the Rio Ribeiro (i.e., Rio Ribeira de Igua- classified as Critically Endangered. Here, we report on pe) into the northern part of the state of Paraná...”. -

Low Paternity Skew and the Influence of Maternal Kin in an Egalitarian
Low paternity skew and the influence of maternal kin in an egalitarian, patrilocal primate Karen B. Striera,1, Paulo B. Chavesb, Sérgio L. Mendesc, Valéria Fagundesc, and Anthony Di Fioreb,d aDepartment of Anthropology, University of Wisconsin, Madison, WI 53706; bDepartment of Anthropology and Center for the Study of Human Origins, New York University, New York, NY 10003; cDepartamento de Ciências Biológicas, Centro de Ciências Humanas e Naturais, Universidade Federal do Espírito Santo, Maruipe, Vitória, ES 29043-900, Brazil; and dDepartment of Anthropology, University of Texas at Austin, Austin, TX 78712 Contributed by Karen B. Strier, October 12, 2011 (sent for review September 11, 2011) Levels of reproductive skew vary in wild primates living in The fecal samples used for paternity analyses included the 22 multimale groups depending on the degree to which high-ranking infants born between 2005 and 2007 that survived to ≥2.08 y of males monopolize access to females. Still, the factors affecting age, their 21 mothers (representing diverse ages and histories) paternity in egalitarian societies remain unexplored. We combine (Table S1), and the 24 adult males that were possible sires of at unique behavioral, life history, and genetic data to evaluate the least one infant (Table S2). We considered a male to be a po- distribution of paternity in the northern muriqui (Brachyteles tential sire for an infant if he was >5 y and was known to have hypoxanthus), a species known for its affiliative, nonhierarchical completed a copulation before the infant’s estimated conception relationships. We genotyped 67 individuals (22 infants born over a date (birth date minus mean 216.4-d gestation) (16). -

Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions
Hindawi International Journal of Rheumatology Volume 2020, Article ID 2919625, 13 pages https://doi.org/10.1155/2020/2919625 Review Article Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions Worku Abie Liyew Biomedical Science Department, School of Medicine, Debre Markos University, Debre Markos, Ethiopia Correspondence should be addressed to Worku Abie Liyew; [email protected] Received 25 April 2020; Revised 26 June 2020; Accepted 13 July 2020; Published 29 August 2020 Academic Editor: Bruce M. Rothschild Copyright © 2020 Worku Abie Liyew. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Lumbar disc degeneration is defined as the wear and tear of lumbar intervertebral disc, and it is mainly occurring at L3-L4 and L4-S1 vertebrae. Lumbar disc degeneration may lead to disc bulging, osteophytes, loss of disc space, and compression and irritation of the adjacent nerve root. Clinical presentations associated with lumbar disc degeneration and lumbosacral nerve lesion are discogenic pain, radical pain, muscular weakness, and cutaneous. Discogenic pain is usually felt in the lumbar region, or sometimes, it may feel in the buttocks, down to the upper thighs, and it is typically presented with sudden forced flexion and/or rotational moment. Radical pain, muscular weakness, and sensory defects associated with lumbosacral nerve lesions are distributed on -

Putting Phylogeny Into the Analysis of Biological Traits: a Methodological Approach Thibaut Jombart, Sandrine Pavoine, Sébastien Devillard, Dominique Pontier
Putting phylogeny into the analysis of biological traits: A methodological approach Thibaut Jombart, Sandrine Pavoine, Sébastien Devillard, Dominique Pontier To cite this version: Thibaut Jombart, Sandrine Pavoine, Sébastien Devillard, Dominique Pontier. Putting phylogeny into the analysis of biological traits: A methodological approach. Journal of Theoretical Biology, Elsevier, 2010, 264 (3), pp.693. 10.1016/j.jtbi.2010.03.038. hal-00591239 HAL Id: hal-00591239 https://hal.archives-ouvertes.fr/hal-00591239 Submitted on 8 May 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Author’s Accepted Manuscript Putting phylogeny into the analysis of biological traits: A methodological approach Thibaut Jombart, Sandrine Pavoine, Sébastien Devillard, Dominique Pontier PII: S0022-5193(10)00173-6 DOI: doi:10.1016/j.jtbi.2010.03.038 Reference: YJTBI5939 www.elsevier.com/locate/yjtbi To appear in: Journal of Theoretical Biology Received date: 20 January 2010 Revised date: 25 March 2010 Accepted date: 25 March 2010 Cite this article as: Thibaut Jombart, Sandrine Pavoine, Sébastien Devillard and Dominique Pontier, Putting phylogeny into the analysis of biological traits: A methodological ap- proach, Journal of Theoretical Biology, doi:10.1016/j.jtbi.2010.03.038 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Differential Evolutionary History in Visual and Olfactory Floral Cues of the Bee-Pollinated Genus Campanula (Campanulaceae)
plants Article Differential Evolutionary History in Visual and Olfactory Floral Cues of the Bee-Pollinated Genus Campanula (Campanulaceae) Paulo Milet-Pinheiro 1,*,† , Pablo Sandro Carvalho Santos 1, Samuel Prieto-Benítez 2,3, Manfred Ayasse 1 and Stefan Dötterl 4 1 Institute of Evolutionary Ecology and Conservation Genomics, University of Ulm, Albert-Einstein Allee, 89081 Ulm, Germany; [email protected] (P.S.C.S.); [email protected] (M.A.) 2 Departamento de Biología y Geología, Física y Química Inorgánica, Universidad Rey Juan Carlos-ESCET, C/Tulipán, s/n, Móstoles, 28933 Madrid, Spain; [email protected] 3 Ecotoxicology of Air Pollution Group, Environmental Department, CIEMAT, Avda. Complutense, 40, 28040 Madrid, Spain 4 Department of Biosciences, Paris-Lodron-University of Salzburg, Hellbrunnerstrasse 34, 5020 Salzburg, Austria; [email protected] * Correspondence: [email protected] † Present address: Universidade de Pernambuco, Campus Petrolina, Rodovia BR 203, KM 2, s/n, Petrolina 56328-900, Brazil. Abstract: Visual and olfactory floral signals play key roles in plant-pollinator interactions. In recent decades, studies investigating the evolution of either of these signals have increased considerably. However, there are large gaps in our understanding of whether or not these two cue modalities evolve in a concerted manner. Here, we characterized the visual (i.e., color) and olfactory (scent) floral cues in bee-pollinated Campanula species by spectrophotometric and chemical methods, respectively, with Citation: Milet-Pinheiro, P.; Santos, the aim of tracing their evolutionary paths. We found a species-specific pattern in color reflectance P.S.C.; Prieto-Benítez, S.; Ayasse, M.; and scent chemistry. -

Bilateral Presence of a Variant Subscapularis Muscle
CASE REPORT Bilateral presence of a variant subscapularis muscle Krause DA and Youdas JW Krause DA, Youdas JW. Bilateral presence of a variant subscapularis the lateral subscapularis inserting into the proximal humerus with the tendon of muscle. Int J Anat Var. 2017;10(4):79-80. the subscapularis. The axillary and lower subscapular nerves coursed between the observed muscle and the subscapularis. The presence of the muscle has potential ABSTRACT to entrap the axillary nerve and the lower subscapular nerve. We describe the presence of an accessory subscapularis muscle observed bilaterally Key Words: Accessory subscapularis; Axillary nerve; Lower subscapular nerve; in a human anatomy laboratory. The muscle originated from the mid-region of Entrapment INTRODUCTION everal anatomic variations in axillary musculature have been reported (1- S4). One of the more common variations is the axillary arch muscle, also known as Langer’s muscle, the axillopectoral muscle, and the pectordorsal muscle (3,4). This muscle typically arises from the lateral aspect of the latissimus dorsi inserting deep to the pectoralis major on the humerus. Several variations of the axillary arch muscle are described with a reported incidence of 6-9% (4-6). Less frequent and distinctly different than the axillary arch muscle, is a variant muscle associated with the subscapularis. This has been termed the subscapulo-humeral muscle, subscapularis minor, subscapularis-teres-latissimus muscle, and the accessory subcapularis muscle (1,2,7). Reported incidence ranges from 0.45 to 2.6% (2,7). CASE REPORT A variant muscle associated with the subscapularis bilaterally was observed on a Caucasian female embalmed cadaver during routine dissection in a human anatomy class for first year physical therapy students. -

Annual Meeting in Tulsa (Hosted by Elmus Beale) on June 11-15, 2019, We Were All Energized
37th ANNUAL Virtual Meeting 2020 June 15-19 President’s Report June 15-19, 2020 Virtual Meeting #AACA Strong Due to the unprecedented COVID-19 pandemic, our 2020 annual AACA meeting in June 15-19 at Weill Cornell in New York City has been canceled. While this is disappointing on many levels, it was an obvious decision (a no brainer for this neurosurgeon) given the current situation and the need to be safe. These past few weeks have been stressful and uncertain for our society, but for all of us personally, professionally and collectively. Through adversity comes opportunity: how we choose to react to this challenge will determine our future. Coming away from the 36th Annual meeting in Tulsa (hosted by Elmus Beale) on June 11-15, 2019, we were all energized. An informative inaugural newsletter edited by Mohammed Khalil was launched in the summer. In the fall, Christina Lewis hosted a successful regional meeting (Augmented Approaches for Incorporating Clinical Anatomy into Education, Research, and Informed Therapeutic Management) with an excellent faculty and nearly 50 attendees at Samuel Merritt University in Oakland, CA. The midyear council meeting was coordinated to overlap with that regional meeting to show solidarity. During the following months, plans for the 2020 New York meeting were well in motion. COVID-19 then surfaced: first with its ripple effect and then its storm. Other societies’ meetings - including AAA and EB – were canceled and outreach to them was extended for them to attend our meeting later in the year. Unfortunately, we subsequently had to cancel the plans for NY. -
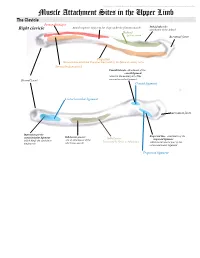
Muscle Attachment Sites in the Upper Limb
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Muscle Attachment Sites in the Upper Limb The Clavicle Pectoralis major Smooth superior surface of the shaft, under the platysma muscle Deltoid tubercle: Right clavicle attachment of the deltoid Deltoid Axillary nerve Acromial facet Trapezius Sternocleidomastoid and Trapezius innervated by the Spinal Accessory nerve Sternocleidomastoid Conoid tubercle, attachment of the conoid ligament which is the medial part of the Sternal facet coracoclavicular ligament Conoid ligament Costoclavicular ligament Acromial facet Impression for the Trapezoid line, attachment of the costoclavicular ligament Subclavian groove: Subclavius trapezoid ligament which binds the clavicle to site of attachment of the Innervated by Nerve to Subclavius which is the lateral part of the the first rib subclavius muscle coracoclavicular ligament Trapezoid ligament This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. The Scapula Trapezius Right scapula: posterior Levator scapulae Supraspinatus Deltoid Deltoid and Teres Minor are innervated by the Axillary nerve Rhomboid minor Levator Scapulae, Rhomboid minor and Rhomboid Major are innervated by the Dorsal Scapular Nerve Supraspinatus and Infraspinatus innervated by the Suprascapular nerve Infraspinatus Long head of triceps Rhomboid major Teres Minor Teres Major Teres Major -

Anatomy of Spinal Nerves in the First Turkish Illustrated Anatomy Handwritten Textbook
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DSpace@HKU Childs Nerv Syst DOI 10.1007/s00381-016-3136-9 COVER EDITORIAL Anatomy of spinal nerves in the first Turkish illustrated anatomy handwritten textbook Murat Çetkin1 & Mustafa Orhan1 & İlhan Bahşi1 & Begümhan Turhan2 Received: 26 May 2016 /Accepted: 30 May 2016 # Springer-Verlag Berlin Heidelberg 2016 BTeşrih-ül Ebdan ve Tercümânı Kıbale-i Feylesûfan^ is the the book, İtâḳî acknowledges the contributions of the Grand first handwritten anatomy textbook with illustrations written Vizier [4, 7]. in Turkish in 17th century by Şemseddîn-i İtâḳî. BTeşrih^ has Not many textbooks about anatomy existed in the Islamic different meanings such as anatomy, skeleton, and cutting a World and the Ottoman Empire until İtâḳî’sbook[9]. In other corpse into pieces [1]. BTeşrih-ül Ebdan ve Tercümânı Kıbale- medical textbooks, anatomy occupies only a few pages in i Feylesûfan ^ means dissection of the body and scholars’ different sections [4]. İtâḳî’s book is a pioneer in its area as birth knowledge [2]. Since this is the first handwritten text- it is written in Turkish, and it is supported with illustrations book in Turkish, it has great importance in the development of [4]. In addition to Turkish, the book contains mostly Arabic medicine in Ottoman Empire. This book was written while and rarely Persian terms as well [4, 6, 7]. Some editions of this Grand Vizier Recep Pasha was in power, and it was dedicated book which was written in the 17th century were reprinted in to the Sultan of that period, Murat the IVth [3, 4]. -

Ecologia De Kerodon Acrobata (Rodentia: Caviidae) Em Fragmentos De Mata Seca Associados a Afloramentos Calcários No Cerrado Do Brasil Central
1 Universidade de Brasília - UnB Instituto de Ciências Biológicas Programa de Pós-Graduação em Ecologia Ecologia de Kerodon acrobata (Rodentia: Caviidae) em fragmentos de mata seca associados a afloramentos calcários no Cerrado do Brasil Central Alexandre de Souza Portella Prof. Dr. Emerson Monteiro Vieira Tese apresentada ao Programa de Pós-Graduação em Ecologia, como requisito parcial para a obtenção do título de Doutor em Ecologia. Brasília, setembro de 2015. 2 3 Sumário Sumário ............................................................................................................................. 3 Agradecimentos ................................................................................................................. 5 Sumário de figuras ............................................................................................................ 7 Sumário de tabelas .......................................................................................................... 10 Sumário de anexos .......................................................................................................... 11 Resumo ............................................................................................................................ 12 Abstract ........................................................................................................................... 14 Introdução geral ............................................................................................................... 16 Referências bibliográficas