Termites (Isoptera) from the Jurassic/Cretaceous Boundary: Evidence for the Longevity of Their Earliest Genera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cómo Citar El Artículo Número Completo Más Información Del
Acta zoológica mexicana ISSN: 0065-1737 ISSN: 2448-8445 Instituto de Ecología A.C. García, Mauricio; Camacho, Jesús; Dorado, Idelma Dos nuevas especies de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Venezuela y observaciones sobre la familia Acta zoológica mexicana, vol. 32, núm. 3, 2016, pp. 348-358 Instituto de Ecología A.C. Disponible en: http://www.redalyc.org/articulo.oa?id=57549165012 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto ISSN 0065-1737 (NUEVA SERIE) 32(3) 2016 DOS NUEVAS ESPECIES DE TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE), DE VENEZUELA Y OBSERVACIONES SOBRE LA FAMILIA TWO NEW SPECIES OF TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE) OF VENEZUELA, AND OBSERVATIONS ON THE FAMILY Mauricio GARCÍA,¹ Jesús CAMACHO² e Idelma DORADO² ¹ Centro de Investigaciones Biológicas (CIB), Facultad de Humanidades y Educación, Edificio de Postgrado, Universidad del Zulia, Apdo. 526, A-4001, Venezuela. ² Museo de Artrópodos de la Universidad del Zulia (MALUZ). Departamento Fitosanitario, Facultad de Agronomía, Universidad del Zulia, Apdo. 526, Maracaibo A-4001, Zulia, Venezuela. <[email protected]>; <[email protected]>; <[email protected]> Recibido: 28/03/2016; aceptado: 30/08/2016 Editor asociado responsable: Alfonso Neri García Aldrete García, M., Camacho, J. & Dorado, I. (2016). Dos nuevas especies García, M., Camacho, J. & Dorado, I. (2016). Two new species of de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae) of Ven- Venezuela y observaciones sobre la familia. -
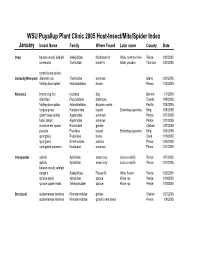
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

André Nel Sixtieth Anniversary Festschrift
Palaeoentomology 002 (6): 534–555 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2019 Magnolia Press Editorial ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.2.6.1 http://zoobank.org/urn:lsid:zoobank.org:pub:25D35BD3-0C86-4BD6-B350-C98CA499A9B4 André Nel sixtieth anniversary Festschrift DANY AZAR1, 2, ROMAIN GARROUSTE3 & ANTONIO ARILLO4 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, P.O. Box: 26110217, Fanar, Matn, Lebanon. Email: [email protected] 2State Key Laboratory of Palaeobiology and Stratigraphy, Center for Excellence in Life and Paleoenvironment, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China. 3Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205-CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP 50, Entomologie, F-75005, Paris, France. 4Departamento de Biodiversidad, Ecología y Evolución, Facultad de Biología, Universidad Complutense, Madrid, Spain. FIGURE 1. Portrait of André Nel. During the last “International Congress on Fossil Insects, mainly by our esteemed Russian colleagues, and where Arthropods and Amber” held this year in the Dominican several of our members in the IPS contributed in edited volumes honoring some of our great scientists. Republic, we unanimously agreed—in the International This issue is a Festschrift to celebrate the 60th Palaeoentomological Society (IPS)—to honor our great birthday of Professor André Nel (from the ‘Muséum colleagues who have given us and the science (and still) national d’Histoire naturelle’, Paris) and constitutes significant knowledge on the evolution of fossil insects a tribute to him for his great ongoing, prolific and his and terrestrial arthropods over the years. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Blattodea: Hodotermitidae) and Its Role As a Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites. -

Preservation of Three-Dimensional Anatomy in Phosphatized Fossil Arthropods Enriches Evolutionary Inference Achim H
Clemson University TigerPrints Publications Plant and Environmental Sciences 2-2016 Preservation of three-dimensional anatomy in phosphatized fossil arthropods enriches evolutionary inference Achim H. Schwermann University of Bonn Tomy dos Santos Rolo Karlsruhe Institute of Technology Michael S. Caterino Clemson University, [email protected] Gunter Bechly State Museum of Natural History Stuttgart, Germany Heiko Schmied University of Bonn See next page for additional authors Follow this and additional works at: https://tigerprints.clemson.edu/ag_pubs Part of the Ecology and Evolutionary Biology Commons Recommended Citation Please use publisher's recommended citation: http://elifesciences.org/content/5/e12129v1/article-info This Article is brought to you for free and open access by the Plant and Environmental Sciences at TigerPrints. It has been accepted for inclusion in Publications by an authorized administrator of TigerPrints. For more information, please contact [email protected]. Authors Achim H. Schwermann, Tomy dos Santos Rolo, Michael S. Caterino, Gunter Bechly, Heiko Schmied, Tilo Baumbach, and Thomas van de Kamp This article is available at TigerPrints: https://tigerprints.clemson.edu/ag_pubs/73 TOOLS AND RESOURCES Preservation of three-dimensional anatomy in phosphatized fossil arthropods enriches evolutionary inference Achim H Schwermann1*, Tomy dos Santos Rolo2, Michael S Caterino3, Gu¨ nter Bechly4, Heiko Schmied5, Tilo Baumbach2,6, Thomas van de Kamp2,6* 1 Steinmann Institute for Geology, Mineralogy and Paleontology, University -

Hemiphlebia Damselfly Version Has Been Prepared for Web Publication
#46 This Action Statement was first published in 1993 and remains current. This Hemiphlebia Damselfly version has been prepared for web publication. It Hemiphlebia mirabilis retains the original text of the action statement, although contact information, the distribution map and the illustration may have been updated. © The State of Victoria, Department of Sustainability and Environment, 2003 Published by the Department of Sustainability and Environment, Victoria. Hemiphlebia Damselfly (Hemiphlebia mirabilis) Distribution in Victoria (DSE 2002) 8 Nicholson Street, East Melbourne, Description and Distribution Victoria 3002 Australia The Hemiphlebia Damselfly (Hemiphlebia Additionally, it was discovered near Mount mirabilis Selys 1868 Odonata: William in Tasmania during 1992 (Trueman This publication may be of Hemiphlebiidae), is a small insect with a et al. 1992) and on Flinders Island in Bass assistance to you but the wingspan of 22 mm and total length of 24 Strait (Endersby 1993). The first Victorian State of Victoria and its employees do not guarantee mm (Davies 1985). It is bright metallic records came from floodplain lagoons in the that the publication is green with white anal appendages (Wells et Goulburn Valley at Alexandra and in the without flaw of any kind or al. 1983). The species was originally middle to upper course of theYarra River at is wholly appropriate for described by Selys in 1868 and more fully the turn of the century (Wells et al. 1983, your particular purposes in 1877 (Yen et al. 1990). Davies 1985). and therefore disclaims all This damselfly is of unusual scientific After continued but declining numbers of liability for any error, loss interest as it exhibits primitive and unique sightings, searchers failed to find the or other consequence which structural features in both adult and larval Hemiphlebia Damselfly on the Goulburn may arise from you relying on any information in this stages (Wells et al. -

Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis
ANRV330-EN53-23 ARI 2 November 2007 18:40 Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis James B. Whitfield1 and Karl M. Kjer2 1Department of Entomology, University of Illinois, Urbana, Illinois 61821; email: jwhitfi[email protected] 2Department of Ecology, Evolution and Natural Resources, Rutgers University, New Brunswick, New Jersey 08901; email: [email protected] Annu. Rev. Entomol. 2008. 53:449–72 Key Words First published online as a Review in Advance on diversification, molecular evolution, Palaeoptera, Orthopteroidea, September 17, 2007 fossils The Annual Review of Entomology is online at ento.annualreviews.org Abstract by UNIVERSITY OF ILLINOIS on 12/18/07. For personal use only. This article’s doi: Phylogenies of major groups of insects based on both morphological 10.1146/annurev.ento.53.103106.093304 and molecular data have sometimes been contentious, often lacking Copyright c 2008 by Annual Reviews. the data to distinguish between alternative views of relationships. Annu. Rev. Entomol. 2008.53:449-472. Downloaded from arjournals.annualreviews.org All rights reserved This paucity of data is often due to real biological and historical 0066-4170/08/0107-0449$20.00 causes, such as shortness of time spans between divergences for evo- lution to occur and long time spans after divergences for subsequent evolutionary changes to obscure the earlier ones. Another reason for difficulty in resolving some of the relationships using molecu- lar data is the limited spectrum of genes so far developed for phy- logeny estimation. For this latter issue, there is cause for current optimism owing to rapid increases in our knowledge of comparative genomics.