The Phylogeny of Termites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mantodea (Insecta), with a Review of Aspects of Functional Morphology and Biology
aua o ew eaa Ramsay, G. W. 1990: Mantodea (Insecta), with a review of aspects of functional morphology and biology. Fauna of New Zealand 19, 96 pp. Editorial Advisory Group (aoimes mae o a oaioa asis MEMBERS AT DSIR PLANT PROTECTION Mou Ae eseac Cee iae ag Aucka ew eaa Ex officio ieco — M ogwo eae Sysemaics Gou — M S ugae Co-opted from within Systematics Group Dr B. A ooway Κ Cosy UIESIIES EESEAIE R. M. Emeso Eomoogy eame ico Uiesiy Caeuy ew eaa MUSEUMS EESEAIE M R. L. ama aua isoy Ui aioa Museum o iae ag Weigo ew eaa OESEAS REPRESENTATIVE J. F. awece CSIO iisio o Eomoogy GO o 1700, Caea Ciy AC 2601, Ausaia Series Editor M C ua Sysemaics Gou SI a oecio Mou Ae eseac Cee iae ag Aucka ew eaa aua o ew eaa Number 19 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy G W Ramsay SI a oecio M Ae eseac Cee iae ag Aucka ew eaa emoa us wig mooogy eosigma cooaio siuaio acousic sesiiiy eece eaiou egeeaio eaio aasiism aoogy a ie Caaoguig-i-uicaio ciaio AMSAY GW Maoea (Iseca – Weigo SI uisig 199 (aua o ew eaa ISS 111-533 ; o 19 IS -77-51-1 I ie II Seies UC 59575(931 Date of publication: see cover of subsequent numbers Suggese om o ciaio amsay GW 199 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy Fauna of New Zealand [no.] 19. —— Fauna o New Zealand is eae o uicaio y e Seies Eio usig comue- ase e ocessig ayou a ase ie ecoogy e Eioia Aisoy Gou a e Seies Eio ackowege e oowig co-oeaio SI UISIG awco – sueisio o oucio a isiuio M C Maews – assisace wi oucio a makeig Ms A Wig – assisace wi uiciy a isiuio MOU AE ESEAC CEE SI Miss M oy -

The Termite by Ogden Nash
Biological studies on two European termite species: establishment risk in the UK Laetitia Virginie Laine B.Sc. M.Sc. D.I.C. A thesis submitted for the degree of Doctor of Philosophy of the University of London November 2002 Department of Biological Sciences, Imperial College, Silwood Park, Ascot, SL5 7PY, Berkshire Abstract The discovery of an accidental introduction of termites into Devon in 1994 generated great interest as termites were previously thought to be unable to establish in the UK due to unfavourable climatic conditions. Information about the species present in Devon, Reticulitermes grassei, was found to be lacking and the present study was undertaken to determine the importance of various abiotic and biotic factors in establishment of this species. The factors included in the study were the minimum termite number for establishment, the consumption of wood and its effect on survival and temperature and soil type. A review of the literature was also conducted, detailing the problems with the taxonomy of this termite genus, their present distribution pattern and the life cycle of Reticulitermes species. Two populations of both R. grassei and R. santonensis were studied. The effect of the minimum termite number was found to be significant in both laboratory and field conditions. However, survival decreased in the laboratory and increased in the field with increased number of termites. Consumption experiments were performed using blocks of Scots pine, beech and oak. In most cases termite populations were found to consume and survive best on oak. Consumption was also tested on live seedlings but these results were inconclusive. Survival was observed to increase with increased temperature. -
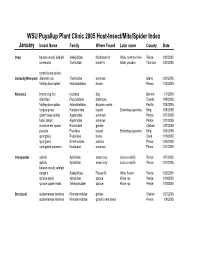
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

The Genus Metallyticus Reviewed (Insecta: Mantodea)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228623877 The genus Metallyticus reviewed (Insecta: Mantodea) Article · September 2008 CITATIONS READS 11 353 1 author: Frank Wieland Pfalzmuseum für Naturkunde - POLLICHIA-… 33 PUBLICATIONS 113 CITATIONS SEE PROFILE All in-text references underlined in blue are linked to publications on ResearchGate, Available from: Frank Wieland letting you access and read them immediately. Retrieved on: 24 October 2016 Species, Phylogeny and Evolution 1, 3 (30.9.2008): 147-170. The genus Metallyticus reviewed (Insecta: Mantodea) Frank Wieland Johann-Friedrich-Blumenbach-Institut für Zoologie & Anthropologie und Zoologisches Museum der Georg-August-Universität, Abteilung für Morphologie, Systematik und Evolutionsbiologie, Berliner Str. 28, 37073 Göttingen, Germany [[email protected]] Abstract Metallyticus Westwood, 1835 (Insecta: Dictyoptera: Mantodea) is one of the most fascinating praying mantids but little is known of its biology. Several morphological traits are plesiomorphic, such as the short prothorax, characters of the wing venation and possibly also the lack of discoidal spines on the fore femora. On the other hand, Metallyticus has autapomor- phies which are unique among extant Mantodea, such as the iridescent bluish-green body coloration and the enlargement of the first posteroventral spine of the fore femora. The present publication reviews our knowledge of Metallyticus thus providing a basis for further research. Data on 115 Metallyticus specimens are gathered and interpreted. The Latin original descriptions of the five Metallyticus species known to date, as well as additional descriptions and a key to species level that were originally published by Giglio-Tos (1927) in French, are translated into English. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands
SYSTEMATICS Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands RUDOLF H. SCHEFFRAHN,1 JAN KRˇ ECˇ EK,1 JAMES A. CHASE,2 BOUDANATH MAHARAJH,1 3 AND JOHN R. MANGOLD Ann. Entomol. Soc. Am. 99(3): 463Ð486 (2006) ABSTRACT Termite surveys of 33 islands of the Bahamas and Turks and Caicos (BATC) archipelago yielded 3,533 colony samples from 593 sites. Twenty-seven species from three families and 12 genera were recorded as follows: Cryptotermes brevis (Walker), Cr. cavifrons Banks, Cr. cymatofrons Schef- Downloaded from frahn and Krˇecˇek, Cr. bracketti n. sp., Incisitermes bequaerti (Snyder), I. incisus (Silvestri), I. milleri (Emerson), I. rhyzophorae Herna´ndez, I. schwarzi (Banks), I. snyderi (Light), Neotermes castaneus (Burmeister), Ne. jouteli (Banks), Ne. luykxi Nickle and Collins, Ne. mona Banks, Procryptotermes corniceps (Snyder), and Pr. hesperus Scheffrahn and Krˇecˇek (Kalotermitidae); Coptotermes gestroi Wasmann, Heterotermes cardini (Snyder), H. sp., Prorhinotermes simplex Hagen, and Reticulitermes flavipes Koller (Rhinotermitidae); and Anoplotermes bahamensis n. sp., A. inopinatus n. sp., Nasuti- termes corniger (Motschulsky), Na. rippertii Rambur, Parvitermes brooksi (Snyder), and Termes http://aesa.oxfordjournals.org/ hispaniolae Banks (Termitidae). Of these species, three species are known only from the Bahamas, whereas 22 have larger regional indigenous ranges that include Cuba, Florida, or Hispaniola and beyond. Recent exotic immigrations for two of the regional indigenous species cannot be excluded. Three species are nonindigenous pests of known recent immigration. IdentiÞcation keys based on the soldier (or soldierless worker) and the winged imago are provided along with species distributions by island. Cr. bracketti, known only from San Salvador Island, Bahamas, is described from the soldier and imago. -

Blattabacterium Genome: Structure, Function, and Evolution Austin Alleman
University of Texas at Tyler Scholar Works at UT Tyler Biology Theses Biology Spring 6-5-2014 Blattabacterium Genome: Structure, Function, and Evolution Austin Alleman Follow this and additional works at: https://scholarworks.uttyler.edu/biology_grad Part of the Biology Commons Recommended Citation Alleman, Austin, "Blattabacterium Genome: Structure, Function, and Evolution" (2014). Biology Theses. Paper 20. http://hdl.handle.net/10950/215 This Thesis is brought to you for free and open access by the Biology at Scholar Works at UT Tyler. It has been accepted for inclusion in Biology Theses by an authorized administrator of Scholar Works at UT Tyler. For more information, please contact [email protected]. BLATTABACTERIUM GENOME: STRUCTURE, FUNCTION, AND EVOLUTION by AUSTIN ALLEMAN A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Biology Srini Kambhampati, Ph. D., Committee Chair College of Arts and Sciences The University of Texas at Tyler May 2014 © Copyright by Austin Alleman 2014 All rights reserved Acknowledgements I would like to thank the Sam A. Lindsey Endowment, without whose support this research would not have been possible. I would also like to thank my research advisor, Dr. Srini Kambhampati, for his knowledge, insight, and patience; and my committee, Dr. John S. Placyk, Jr. and Dr. Blake Bextine, for their assistance and feedback. “It is the supreme art of the teacher to awaken joy in creative expression and knowledge.” --Albert Einstein Table of Contents Chapter -

197 Section 9 Sunflower (Helianthus
SECTION 9 SUNFLOWER (HELIANTHUS ANNUUS L.) 1. Taxonomy of the Genus Helianthus, Natural Habitat and Origins of the Cultivated Sunflower A. Taxonomy of the genus Helianthus The sunflower belongs to the genus Helianthus in the Composite family (Asterales order), which includes species with very diverse morphologies (herbs, shrubs, lianas, etc.). The genus Helianthus belongs to the Heliantheae tribe. This includes approximately 50 species originating in North and Central America. The basis for the botanical classification of the genus Helianthus was proposed by Heiser et al. (1969) and refined subsequently using new phenological, cladistic and biosystematic methods, (Robinson, 1979; Anashchenko, 1974, 1979; Schilling and Heiser, 1981) or molecular markers (Sossey-Alaoui et al., 1998). This approach splits Helianthus into four sections: Helianthus, Agrestes, Ciliares and Atrorubens. This classification is set out in Table 1.18. Section Helianthus This section comprises 12 species, including H. annuus, the cultivated sunflower. These species, which are diploid (2n = 34), are interfertile and annual in almost all cases. For the majority, the natural distribution is central and western North America. They are generally well adapted to dry or even arid areas and sandy soils. The widespread H. annuus L. species includes (Heiser et al., 1969) plants cultivated for seed or fodder referred to as H. annuus var. macrocarpus (D.C), or cultivated for ornament (H. annuus subsp. annuus), and uncultivated wild and weedy plants (H. annuus subsp. lenticularis, H. annuus subsp. Texanus, etc.). Leaves of these species are usually alternate, ovoid and with a long petiole. Flower heads, or capitula, consist of tubular and ligulate florets, which may be deep purple, red or yellow. -

Senator 600 Version: 17 November 2014 Page 1 of 6 ______
Senator 600 Version: 17 November 2014 Page 1 of 6 ________________________________________________________________________________________________ POISON KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS BEFORE OPENING OR USING ® SENATOR 600 FLOWABLE SEED TREATMENT ACTIVE CONSTITUENT: 600 g/L IMIDACLOPRID GROUP 4A INSECTICIDE 4A Seed dressing for the control of various insect pests in a range of crops and the prevention of spread of barley yellow dwarf virus in cereal crops and for protection against insect pests of stored seed grain as specified in the Directions for Use table. FOR USE BY PROFESSIONAL SEED TREATMENT COMPANIES ONLY CONTENTS: 10L, 100L, 200L and 1000L Crop Care Australasia Pty Ltd, Unit 17/16 Metroplex Avenue, Murarrie Qld 4172 Senator 600 Version: 17 November 2014 Page 2 of 6 ________________________________________________________________________________________________ DIRECTIONS FOR USE See GENERAL INSTRUCTIONS for specific application details. CROP PEST RATE CRITICAL COMMENTS Cotton Thrips 580 mL, 875 Thrip damage is dependent upon thrips mL or 1.17 L infesting cotton seedlings and the /100kg of seed subsequent growth rate of the plants. Choose a higher rate if high thrip pressure is expected (e.g. winter cereals and weeds supporting thrips) and/or cotton seedlings are expected to experience slow growth (e.g. cool weather from early planting or sown in shorter season districts). The mid-rate is considered a general rate for normal conditions. Brown flea beetle When applied for thrip control, these rates will also reduce damage to cotyledons caused by brown flea beetle. Aphids 875 mL or When applied for thrip control, these rates 1.17 L/100kg will also control early season aphids. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Blattodea: Hodotermitidae) and Its Role As a Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites.