Order Blattodea*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ancient Roaches Further Exemplify 'No Land Return' in Aquatic Insects
Gondwana Research 68 (2019) 22–33 Contents lists available at ScienceDirect Gondwana Research journal homepage: www.elsevier.com/locate/gr Ancient roaches further exemplify ‘no land return’ in aquatic insects Peter Vršanský a,b,c,d,1, Hemen Sendi e,⁎,1, Danil Aristov d,f,1, Günter Bechly g,PatrickMüllerh, Sieghard Ellenberger i, Dany Azar j,k, Kyoichiro Ueda l, Peter Barna c,ThierryGarciam a Institute of Zoology, Slovak Academy of Sciences, Dúbravská cesta 9, 845 06 Bratislava, Slovakia b Slovak Academy of Sciences, Institute of Physics, Research Center for Quantum Information, Dúbravská cesta 9, Bratislava 84511, Slovakia c Earth Science Institute, Slovak Academy of Sciences, Dúbravská cesta 9, P.O. BOX 106, 840 05 Bratislava, Slovakia d Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya 123, 117868 Moscow, Russia e Faculty of Natural Sciences, Comenius University, Ilkovičova 6, Bratislava 84215, Slovakia f Cherepovets State University, Cherepovets 162600, Russia g Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, D-70191 Stuttgart, Germany h Friedhofstraße 9, 66894 Käshofen, Germany i Bodelschwinghstraße 13, 34119 Kassel, Germany j State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, PR China k Lebanese University, Faculty of Science II, Fanar, Natural Sciences Department, PO Box 26110217, Fanar - Matn, Lebanon l Kitakyushu Museum, Japan m River Bigal Conservation Project, Avenida Rafael Andrade y clotario Vargas, 220450 Loreto, Orellana, Ecuador article info abstract Article history: Among insects, 236 families in 18 of 44 orders independently invaded water. We report living amphibiotic cock- Received 13 July 2018 roaches from tropical streams of UNESCO BR Sumaco, Ecuador. -
Host Insect List 2005
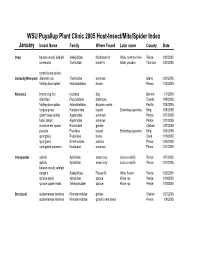
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Termites (Isoptera) in the Azores: an Overview of the Four Invasive Species Currently Present in the Archipelago
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago MARIA TERESA FERREIRA ET AL. Ferreira, M.T., P.A.V. Borges, L. Nunes, T.G. Myles, O. Guerreiro & R.H. Schef- frahn 2013. Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago. Arquipelago. Life and Marine Sciences 30: 39-55. In this contribution we summarize the current status of the known termites of the Azores (North Atlantic; 37-40° N, 25-31° W). Since 2000, four species of termites have been iden- tified in the Azorean archipelago. These are spreading throughout the islands and becoming common structural and agricultural pests. Two termites of the Kalotermitidae family, Cryp- totermes brevis (Walker) and Kalotermes flavicollis (Fabricius) are found on six and three of the islands, respectively. The other two species, the subterranean termites Reticulitermes grassei Clemént and R. flavipes (Kollar) of the Rhinotermitidae family are found only in confined areas of the cities of Horta (Faial) and Praia da Vitória (Terceira) respectively. Due to its location and weather conditions the Azorean archipelago is vulnerable to coloni- zation by invasive species. The fact that there are four different species of termites in the Azores, all of them considered pests, is a matter of concern. Here we present a comparative description of these species, their known distribution in the archipelago, which control measures are being used against them, and what can be done in the future to eradicate and control these pests in the Azores. -

Blattodea: Hodotermitidae) and Its Role As a Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites. -

The Control of Turkestan Cockroach Blatta Lateralis (Dictyoptera: Blattidae)
Türk Tarım ve Doğa Bilimleri Dergisi 7(2): 375-380, 2020 https://doi.org/10.30910/turkjans.725807 TÜRK TURKISH TARIM ve DOĞA BİLİMLERİ JOURNAL of AGRICULTURAL DERGİSİ and NATURAL SCIENCES www.dergipark.gov.tr/turkjans Research Article The Control of Turkestan Cockroach Blatta lateralis (Dictyoptera: Blattidae) by The Entomopathogenic nematode Heterorhabditis bacteriophora HBH (Rhabditida: Heterorhabditidae) Using Hydrophilic Fabric Trap Yavuz Selim ŞAHİN, İsmail Alper SUSURLUK* Bursa Uludağ University, Faculty of Agriculture, Department of Plant Protection, 16059, Nilüfer, Bursa, Turkey *Corresponding author: [email protected] Receieved: 09.09.2019 Revised in Received: 18.02.2020 Accepted: 19.02.2020 Abstract Chemical insecticides used against cockroaches, which are an important urban pest and considered public health, are harmful to human health and cause insects to gain resistance. The entomopathogenic nematode (EPN), Heterorhabditis bacteriophora HBH, were used in place of chemical insecticides within the scope of biological control against the Turkestan cockroaches Blatta lateralis in this study. The hydrophilic fabric traps were set to provide the moist environment needed by the EPNs on aboveground. The fabrics inoculated with the nematodes at 50, 100 and 150 IJs/cm2 were used throughout the 37-day experiment. The first treatment was performed by adding 10 adult cockroaches immediately after the establishment of the traps. In the same way, the second treatment was applied after 15 days and the third treatment after 30 days. The mortality rates of cockroaches after 4 and 7 days of exposure to EPNs were determined for all treatments. Although Turkestan cockroaches were exposed to HBH 30 days after the setting of the traps, infection occurred. -

US EPA, Pesticide Product Label, PYRIPROXYFEN 10% EC,06/24
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION June 24, 2021 Lisa Adamson Regulatory Manager Control Solutions, Inc. 5903 Genoa-Red Bluff Pasadena, TX 77507-1041 Subject: Registration Review Label Mitigation for Pyriproxyfen Product Name: Pyriproxyfen 10% EC EPA Registration Number: 53883-280 Application Date: 02/21/2020 Decision Number: 560323 Dear Ms. Adamson: The Agency, in accordance with the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), as amended, has completed reviewing all the information submitted with your application to support the Registration Review of the above referenced product in connection with the Pyriproxyfen Interim Decision, and has concluded that your submission is acceptable. The label referred to above, submitted in connection with registration under FIFRA, as amended, is acceptable. Should you wish to add/retain a reference to the company’s website on your label, then please be aware that the website becomes labeling under the Federal Insecticide Fungicide and Rodenticide Act and is subject to review by the Agency. If the website is false or misleading, the product would be misbranded and unlawful to sell or distribute under FIFRA section 12(a)(1)(E). 40 CFR 156.10(a)(5) list examples of statements EPA may consider false or misleading. In addition, regardless of whether a website is referenced on your product’s label, claims made on the website may not substantially differ from those claims approved through the registration process. Therefore, should the Agency find or if it is brought to our attention that a website contains false or misleading statements or claims substantially differing from the EPA approved registration, the website will be referred to the EPA’s Office of Enforcement and Compliance. -

RESEARCH ARTICLE a New Species of Cockroach, Periplaneta
Tropical Biomedicine 38(2): 48-52 (2021) https://doi.org/10.47665/tb.38.2.036 RESEARCH ARTICLE A new species of cockroach, Periplaneta gajajimana sp. nov., collected in Gajajima, Kagoshima Prefecture, Japan Komatsu, N.1, Iio, H.2, Ooi, H.K.3* 1Civil International Corporation, 10–14 Kitaueno 1, Taito–ku, Tokyo, 110–0014, Japan 2Foundation for the Protection of Deer in Nara, 160-1 Kasugano-cho, Nara-City, Nara, 630-8212, Japan 3Laboratory of Parasitology, School of Veterinary Medicine, Azabu University, 1-17-710 Fuchinobe, Sagamihara, Kanagawa 252-5201 Japan *Corresponding author: [email protected] ARTICLE HISTORY ABSTRACT Received: 25 January 2021 We described a new species of cockroach, Periplaneta gajajimana sp. nov., which was collected Revised: 2 February 2021 in Gajajima, Kagoshima-gun Toshimamura, Kagoshima Prefecture, Japan, on November 2012. Accepted: 2 February 2021 The new species is characterized by its reddish brown to blackish brown body, smooth Published: 30 April 2021 surface pronotum, well developed compound eyes, dark brown head apex, dark reddish brown front face and small white ocelli connected to the antennal sockets. In male, the tegmen tip reach the abdomen end or are slightly shorter, while in the female, it does not reach the abdominal end and exposes the abdomen beyond the 7th abdominal plate. We confirmed the validity of this new species by breeding the specimens in our laboratory to demonstrate that the features of the progeny were maintained for several generations. For comparison and easy identification of this new species, the key to species identification of the genus Periplaneta that had been reported in Japan to date are also presented. -

The Phylogeny of Termites
Molecular Phylogenetics and Evolution 48 (2008) 615–627 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: Implications for the evolution of the worker and pseudergate castes, and foraging behaviors Frédéric Legendre a,*, Michael F. Whiting b, Christian Bordereau c, Eliana M. Cancello d, Theodore A. Evans e, Philippe Grandcolas a a Muséum national d’Histoire naturelle, Département Systématique et Évolution, UMR 5202, CNRS, CP 50 (Entomologie), 45 rue Buffon, 75005 Paris, France b Department of Integrative Biology, 693 Widtsoe Building, Brigham Young University, Provo, UT 84602, USA c UMR 5548, Développement—Communication chimique, Université de Bourgogne, 6, Bd Gabriel 21000 Dijon, France d Muzeu de Zoologia da Universidade de São Paulo, Avenida Nazaré 481, 04263-000 São Paulo, SP, Brazil e CSIRO Entomology, Ecosystem Management: Functional Biodiversity, Canberra, Australia article info abstract Article history: A phylogenetic hypothesis of termite relationships was inferred from DNA sequence data. Seven gene Received 31 October 2007 fragments (12S rDNA, 16S rDNA, 18S rDNA, 28S rDNA, cytochrome oxidase I, cytochrome oxidase II Revised 25 March 2008 and cytochrome b) were sequenced for 40 termite exemplars, representing all termite families and 14 Accepted 9 April 2008 outgroups. Termites were found to be monophyletic with Mastotermes darwiniensis (Mastotermitidae) Available online 27 May 2008 as sister group to the remainder of the termites. In this remainder, the family Kalotermitidae was sister group to other families. The families Kalotermitidae, Hodotermitidae and Termitidae were retrieved as Keywords: monophyletic whereas the Termopsidae and Rhinotermitidae appeared paraphyletic. -

Novel Lineages of Oxymonad Flagellates from the Termite Porotermes Adamsoni (Stolotermitidae): the Genera Oxynympha and Termitim
Protist, Vol. 170, 125683, December 2019 http://www.elsevier.de/protis Published online date 21 October 2019 ORIGINAL PAPER Novel Lineages of Oxymonad Flagellates from the Termite Porotermes adamsoni (Stolotermitidae): the Genera Oxynympha and Termitimonas a,1 b a c b,1 Renate Radek , Katja Meuser , Samet Altinay , Nathan Lo , and Andreas Brune a Evolutionary Biology, Institute for Biology/Zoology, Freie Universität Berlin, 14195 Berlin, Germany b Research Group Insect Gut Microbiology and Symbiosis, Max Planck Institute for Terrestrial Microbiology, 35043 Marburg, Germany c School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW 2006, Australia Submitted January 21, 2019; Accepted October 9, 2019 Monitoring Editor: Alastair Simpson The symbiotic gut flagellates of lower termites form host-specific consortia composed of Parabasalia and Oxymonadida. The analysis of their coevolution with termites is hampered by a lack of informa- tion, particularly on the flagellates colonizing the basal host lineages. To date, there are no reports on the presence of oxymonads in termites of the family Stolotermitidae. We discovered three novel, deep-branching lineages of oxymonads in a member of this family, the damp-wood termite Porotermes adamsoni. One tiny species (6–10 m), Termitimonas travisi, morphologically resembles members of the genus Monocercomonoides, but its SSU rRNA genes are highly dissimilar to recently published sequences of Polymastigidae from cockroaches and vertebrates. A second small species (9–13 m), Oxynympha loricata, has a slight phylogenetic affinity to members of the Saccinobaculidae, which are found exclusively in wood-feeding cockroaches of the genus Cryptocercus, the closest relatives of termites, but shows a combination of morphological features that is unprecedented in any oxymonad family.