Insect Pathogens As Biological Control Agents: Back to the Future ⇑ L.A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
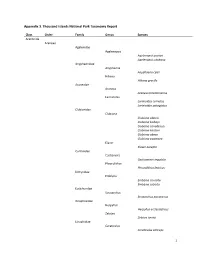
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Baculovirus Nuclear Import: Open, Nuclear Pore Complex (NPC) Sesame
Viruses 2013, 5, 1885-1900; doi:10.3390/v5071885 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Baculovirus Nuclear Import: Open, Nuclear Pore Complex (NPC) Sesame Shelly Au, Wei Wu and Nelly Panté * Department of Zoology, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia V6T 1Z4, Canada; E-Mails: [email protected] (S.A.); [email protected] (W.W.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-604-822-3369; Fax: +1-604-822-2416. Received: 30 May 2013; in revised form: 17 July 2013 / Accepted: 17 July 2013 / Published: 23 July 2013 Abstract: Baculoviruses are one of the largest viruses that replicate in the nucleus of their host cells. During infection, the rod-shape, 250-nm long nucleocapsid delivers its genome into the nucleus. Electron microscopy evidence suggests that baculoviruses, specifically the Alphabaculoviruses (nucleopolyhedroviruses) and the Betabaculoviruses (granuloviruses), have evolved two very distinct modes for doing this. Here we review historical and current experimental results of baculovirus nuclear import studies, with an emphasis on electron microscopy studies employing the prototypical baculovirus Autographa californica multiple nucleopolyhedrovirus infecting cultured cells. We also discuss the implications of recent studies towards theories of nuclear transport mechanisms. Keywords: baculovirus; AcMNPV; nuclear import; nuclear pore complex; viruses 1. Introduction Baculoviruses are a large and diverse group of rod-shaped, enveloped, double-stranded DNA viruses that replicate in the nucleus of their host cells. They are pathogenic to arthropods, mainly insects, and are ubiquitously found in the environment. Members of the Baculoviridae family have been isolated from more than 700 host species. -

Autographa Gamma
1 Table of Contents Table of Contents Authors, Reviewers, Draft Log 4 Introduction to the Reference 6 Soybean Background 11 Arthropods 14 Primary Pests of Soybean (Full Pest Datasheet) 14 Adoretus sinicus ............................................................................................................. 14 Autographa gamma ....................................................................................................... 26 Chrysodeixis chalcites ................................................................................................... 36 Cydia fabivora ................................................................................................................. 49 Diabrotica speciosa ........................................................................................................ 55 Helicoverpa armigera..................................................................................................... 65 Leguminivora glycinivorella .......................................................................................... 80 Mamestra brassicae....................................................................................................... 85 Spodoptera littoralis ....................................................................................................... 94 Spodoptera litura .......................................................................................................... 106 Secondary Pests of Soybean (Truncated Pest Datasheet) 118 Adoxophyes orana ...................................................................................................... -

Establishment and Characterization of Insect Cell Lines from 10 Lepidopteran Species
In Vitro Cell. Dev. Biol.ÐAnimal 37:367±373, June 2001 q 2001 Society for In Vitro Biology 1071-2690/01 $10.00+0.00 ESTABLISHMENT AND CHARACTERIZATION OF INSECT CELL LINES FROM 10 LEPIDOPTERAN SPECIES CYNTHIA L. GOODMAN,1 GALAL N. EL SAYED, ARTHUR H. MCINTOSH, JAMES J. GRASELA, AND BRAD STILES Biological Control of Insects Research Laboratory (BCIRL), U.S. Department of Agriculture (USDA),2 Agricultural Research Service (ARS), Columbia, Missouri 65203-3535 (C. L. G., A. H. M., J. J. G.), Department of Entomology, University of Missouri, Columbia, Missouri 65211 (G. N. E.), and BASF Agro Research (formerly American Cyanamid Co., Agricultural Research Div.), P.O. Box 400, Princeton, New Jersey 08540 (B. S.) (Received 27 December 2000; accepted 16 March 2001) SUMMARY Cell lines from selected lepidopteran species were established for the overall purpose of use in baculovirus production. A total of 36 new cell lines from 10 lepidopteran species were generated, including cell lines from a pyralid, the European corn borer, Ostrinia nubilalis, a plutellid, the diamondback moth, Plutella xylostella, as well as eight noctuids: the black cutworm, Agrotis ipsilon, the celery looper, Anagrapha falcifera, the velvetbean caterpillar, Anticarsia gemmatalis, the corn earworm, Helicoverpa zea, the tobacco budworm, Heliothis virescens, the beet armyworm, Spodoptera exigua, the fall armyworm, Spodoptera frugiperda, and the cabbage looper, Trichoplusia ni. Tissues used for cell line establishment included fat bodies, ovaries, testes, or whole embryos/larvae/pupae. All the cell lines were subcultured numerous times, characterized by isoenzyme analysis and/or deoxyribonucleic acid ampli®cation ®ngerprinting using polymerase chain reaction, and stored in liquid nitrogen. -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

I Ohten Deutsche Gesellschaft Für Allgemeine Und Angewandte Entomologie E.V., Ulm 6
i ohten Deutsche Gesellschaft für allgemeine und angewandte Entomologie e.V., Ulm 6. Jahrgang, Heft 1 ISSN 0931-4873 Februar 1992 INHALTSVERZEICHNIS AUS DEN ARBEITSKREISEN: AK "Nutzarthropoden", S. 2; AK "Dipteren", S. 13; Ankündigungen der AKe "Dipteren", S. 20; Einladung AK "Nutzarthropoden", S. 21; Einladung AK "Epigäische Raubarthropoden", S. 22; ÜBERSICHTEN ÜBER ENTOMOLOGISCHE ARBEITSGRUPPEN: Limnologische Flußstation Schlitz des MPI für Limnologie, S. 23; BITTE UM MITARBEIT (Staphylinidologen), S. 28; TAGUNGEN, S. 30; GESELLSCHAFTSNACHRICHTEN: Meigen-Medaille, S. 32; Neue Mitglieder, S. 37; Ehrenmitglieder, S. 38; Austritte 1991 und in 1991 ver- storbene Mitglieder, S. 38; Spendenbescheinigung, S. 39; Anschriftenänderun- gen; Mitgliedsbeiträge, Konten, Impressum, S. 40. !!! Studentische Mitglieder !!! Bitte unbedingt eine Studienbescheinigung an den Kassenwart senden, sofern nicht bereits im Wintersemester 1991/1992 geschehen. Wenn keine Studienbe- scheinigung vorliegt, muß der volle Mitgliedsbeitrag berechnet werden. Bitte beachten: Neue Anschrift des Kassenwartes: Dr. Paul Bernhard Koch Aligemeine Zoologie (Biologie I) Albert-Einstein-Allee 11 7900 Ulm Tel. 0731/502-2600, FAX 0731/502-2038 AUS DEN ARBEITSKREISEN Arbeitskreis "Nutzarthropoden" Die 10. Tagung des Arbeitskreises "Nutzarthropoden" fand am 18. und 19. Sep- tember 1991 im Landwirtschaftsamt, Karlsruhe-Durlach (Augustenberg) statt. Gastgeber waren Herr Amtsleiter A. BEEG und Herr P. DETZEL, Beratungsdienst "Nützlingseinsatz". Ungefähr 50 Kollegen nahmen Teil. Dabei wurden -

Report on the Badlands/Parkland Lepidoptera Survey 2017 by the Alberta Lepidopterists' Guild, Under Research Permit #17-171
Report on the Badlands/Parkland Lepidoptera Survey 2017 by the Alberta Lepidopterists' Guild, under research permit #17-171 Report to Alberta Tourism, Park and Recreation, Parks Division November 2017 by Gregory R. Pohl Gregory Pohl and other members of the Alberta Lepidopterists' Guild were granted a research permit (#17-171) for moth and butterfly (Lepidoptera) observation and collection in the Tolman - Rumsey area of central Alberta in the summer of 2017. This is our report of the species observed and collected in the area. Study Sites: The following sites were visited and sampled for Lepidoptera: 1. Rowley townsite (Figure 1). 51.760°N 112.786°W. July 14-16, 2017. Abandoned home sites and field margins; disturbed area along train tracks. Although not a protected area requiring a permit, this was our base of operations and camping area, it was convenient to observe and collect moths and butterflies here. Most of the species encountered here are expected to occur in nearby parks and natural areas. Collecting methods - daytime observation and netting; UV light traps; mercury vapour lights. 2. "North Rumsey": Township Road 589, vicinity of Rumsey Natural Area. 51.965°N 112.625°W. July 15, 2017. Rolling parkland with small sloughs. Although not technically within the Rumsey Natural Area, this site is very near and is comprised of similar habitat. The species seen here are all expected within the natural area. Collecting methods - daytime observation and netting. 3. "West Rumsey": Western edge of Rumsey Natural Area (Figure 2). 51.882°N 112.691°W. July 15, 2017. Rolling parkland and grassland. -

Malaise-Hyönteispyynti Lapin Suojelualueilla 2012–2014
Jukka Salmela, Stefan Siivonen, Patrycja Dominiak, Antti Haarto, Kai Heller, Juhani Kanervo, Petri Martikainen, Matti Mäkilä, Lauri Paasivirta, Aki Rinne, Juha Salokannel, Guy Söderman ja Pekka Vilkamaa Malaise-hyönteispyynti Lapin suojelualueilla 2012–2014 Metsähallituksen luonnonsuojelujulkaisuja. Sarja A 221 Jukka Salmela, Metsähallitus, Lapin luontopalvelut, jukka.salmela(at)metsa.fi Stefan Siivonen, Metsähallitus, Lapin luontopalvelut, stefan.siivonen(at)metsa.fi Patrycja Dominiak, Department of Invertebrate Zoology and Parasitology, University of Gdansk, heliocopris(at)gmail.com Antti Haarto, Mietoinen, ahaarto(at)gmail.com Kai Heller, Quickborn, kaiheller(at)gmx.de Juhani Kanervo, Turku, jussi.kanervo(at)luukku.com Petri Martikainen, Juva, petri.martikainen(at)uef.fi Matti Mäkilä, Rovaniemi, makila.entomology(at)gmail.com Lauri Paasivirta, Salo, lauri.paasivirta(at)suomi24.fi Aki Rinne, Helsinki, aki.rinne(at)pintakasittelytekniikka.fi Juha Salokannel, Tampere, juha.salokannel(at)gmail.com Guy Söderman, Helsinki, guy.soderman(at)pp.inet.fi Pekka Vilkamaa, Luonnontieteellinen keskusmuseo, Helsingin yliopisto, pekka.vilkamaa(at)helsinki.fi Kansikuva: Malaise-pyydys Pallas–Yllästunturin kansallispuiston Röyninkurussa 2013. Lähteisten latvapurojen varret, varsinkin sellaiset joita ympäröi luonnontilainen havu- metsä, ovat monimuotoisia elinympäristöjä. Tältä paikalta havaittiin mm. Euroopalle uusi sienissääskilaji Mycetophila monstera, erittäin harvinainen pikkuvaaksiainen ou- taruskokirsikäs (Limonia messaurea) ja pohjoinen surviaissääski -

The Isolation and Genetic Characterisation of a Novel Alphabaculovirus for the Microbial Control of Cryptophlebia Peltastica and Closely Related Tortricid Pests
RHODES UNIVERSITY Where leaders learn The isolation and genetic characterisation of a novel alphabaculovirus for the microbial control of Cryptophlebia peltastica and closely related tortricid pests Submitted in fulfilment of the requirements for the degree of DOCTOR OF PHILOSOPHY At RHODES UNIVERSITY By TAMRYN MARSBERG December 2016 ABSTRACT Cryptophlebia peltastica (Meyrick) (Lepidoptera: Tortricidae) is an economically damaging pest of litchis and macadamias in South Africa. Cryptophlebia peltastica causes both pre- and post-harvest damage to litchis, reducing overall yields and thus classifying the pest as a phytosanitary risk. Various control methods have been implemented against C. peltastica in an integrated pest management programme. These control methods include chemical control, cultural control and biological control. However, these methods have not yet provided satisfactory control as of yet. As a result, an alternative control option needs to be identified and implemented into the IPM programme. An alternative method of control that has proved successful in other agricultural sectors and not yet implemented in the control of C. peltastica is that of microbial control, specifically the use of baculovirus biopesticides. This study aimed to isolate and characterise a novel baculovirus from a laboratory culture of C. peltastica that could be used as a commercially available baculovirus biopesticide. In order to isolate a baculovirus a laboratory culture of C. peltastica was successfully established at Rhodes University, Grahamstown, South Africa. This is the first time a laboratory culture of C. peltastica has been established. This allowed for various biological aspects of the pest to be determined, which included: length of the life cycle, fecundity and time to oviposition, egg and larval development and percentage hatch. -

Spiders in Africa - Hisham K
ANIMAL RESOURCES AND DIVERSITY IN AFRICA - Spiders In Africa - Hisham K. El-Hennawy SPIDERS IN AFRICA Hisham K. El-Hennawy Arachnid Collection of Egypt, Cairo, Egypt Keywords: Spiders, Africa, habitats, behavior, predation, mating habits, spiders enemies, venomous spiders, biological control, language, folklore, spider studies. Contents 1. Introduction 1.1. Africa, the continent of the largest web spinning spider known 1.2. Africa, the continent of the largest orb-web ever known 2. Spiders in African languages and folklore 2.1. The names for “spider” in Africa 2.2. Spiders in African folklore 2.3. Scientific names of spider taxa derived from African languages 3. How many spider species are recorded from Africa? 3.1. Spider families represented in Africa by 75-100% of world species 3.2. Spider families represented in Africa by more than 400 species 4. Where do spiders live in Africa? 4.1. Agricultural lands 4.2. Deserts 4.3. Mountainous areas 4.4. Wetlands 4.5. Water spiders 4.6. Spider dispersal 4.7. Living with others – Commensalism 5. The behavior of spiders 5.1. Spiders are predatory animals 5.2. Mating habits of spiders 6. Enemies of spiders 6.1. The first case of the species Pseudopompilus humboldti: 6.2. The second case of the species Paracyphononyx ruficrus: 7. Development of spider studies in Africa 8. Venomous spiders of Africa 9. BeneficialUNESCO role of spiders in Africa – EOLSS 10. Conclusion AcknowledgmentsSAMPLE CHAPTERS Glossary Bibliography Biographical Sketch Summary There are 7935 species, 1116 genera, and 79 families of spiders recorded from Africa. This means that more than 72% of the known spider families of the world are represented in the continent, while only 19% of the described spider species are ©Encyclopedia of Life Support Systems (EOLSS) ANIMAL RESOURCES AND DIVERSITY IN AFRICA - Spiders In Africa - Hisham K. -

Effect of Trap Color on Captures of Bark- and Wood-Boring Beetles
insects Article Effect of Trap Color on Captures of Bark- and Wood-Boring Beetles (Coleoptera; Buprestidae and Scolytinae) and Associated Predators Giacomo Cavaletto 1,*, Massimo Faccoli 1, Lorenzo Marini 1 , Johannes Spaethe 2 , Gianluca Magnani 3 and Davide Rassati 1,* 1 Department of Agronomy, Food, Natural Resources, Animals and Environment (DAFNAE), University of Padova, Viale dell’Università, 16–35020 Legnaro, Italy; [email protected] (M.F.); [email protected] (L.M.) 2 Department of Behavioral Physiology & Sociobiology, Biozentrum, University of Würzburg, Am Hubland, 97074 Würzburg, Germany; [email protected] 3 Via Gianfanti 6, 47521 Cesena, Italy; [email protected] * Correspondence: [email protected] (G.C.); [email protected] (D.R.); Tel.: +39-049-8272875 (G.C.); +39-049-8272803 (D.R.) Received: 9 October 2020; Accepted: 28 October 2020; Published: 30 October 2020 Simple Summary: Several wood-associated insects are inadvertently introduced every year within wood-packaging materials used in international trade. These insects can cause impressive economic and ecological damage in the invaded environment. Thus, several countries use traps baited with pheromones and plant volatiles at ports of entry and surrounding natural areas to intercept incoming exotic species soon after their arrival and thereby reduce the likelihood of their establishment. In this study, we investigated the performance of eight trap colors in attracting jewel beetles and bark and ambrosia beetles to test if the trap colors currently used in survey programs worldwide are the most efficient for trapping these potential forest pests. In addition, we tested whether trap colors can be exploited to minimize inadvertent removal of their natural enemies.