Cranberry Girdler
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
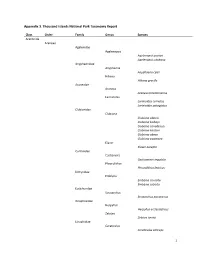
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Report on the Badlands/Parkland Lepidoptera Survey 2017 by the Alberta Lepidopterists' Guild, Under Research Permit #17-171
Report on the Badlands/Parkland Lepidoptera Survey 2017 by the Alberta Lepidopterists' Guild, under research permit #17-171 Report to Alberta Tourism, Park and Recreation, Parks Division November 2017 by Gregory R. Pohl Gregory Pohl and other members of the Alberta Lepidopterists' Guild were granted a research permit (#17-171) for moth and butterfly (Lepidoptera) observation and collection in the Tolman - Rumsey area of central Alberta in the summer of 2017. This is our report of the species observed and collected in the area. Study Sites: The following sites were visited and sampled for Lepidoptera: 1. Rowley townsite (Figure 1). 51.760°N 112.786°W. July 14-16, 2017. Abandoned home sites and field margins; disturbed area along train tracks. Although not a protected area requiring a permit, this was our base of operations and camping area, it was convenient to observe and collect moths and butterflies here. Most of the species encountered here are expected to occur in nearby parks and natural areas. Collecting methods - daytime observation and netting; UV light traps; mercury vapour lights. 2. "North Rumsey": Township Road 589, vicinity of Rumsey Natural Area. 51.965°N 112.625°W. July 15, 2017. Rolling parkland with small sloughs. Although not technically within the Rumsey Natural Area, this site is very near and is comprised of similar habitat. The species seen here are all expected within the natural area. Collecting methods - daytime observation and netting. 3. "West Rumsey": Western edge of Rumsey Natural Area (Figure 2). 51.882°N 112.691°W. July 15, 2017. Rolling parkland and grassland. -

Are Adult Crambid Snout Moths (Crambinae)
Insects 2011, 2, 400-411; doi:10.3390/insects2030400 OPEN ACCESS insects ISSN 2075-4450 www.mdpi.com/journal/insects/ Article Are Adult Crambid Snout Moths (Crambinae) and Larval Stages of Lepidoptera Suitable Tools for an Environmental Monitoring of Transgenic Crops? — Implications of a Field Test 1, 2 3 2 Andreas Lang *, Matthias Dolek , Bernhard Theißen and Andreas Zapp 1 Institute of Environmental Geosciences, University of Basel, Bernoullistrasse 30, Basel CH-4056, Switzerland 2 Büro Geyer & Dolek, Obere Dorfstr. 16, Wörthsee D-82237, Germany; E-Mails: [email protected] (M.D.); [email protected] (A.Z.) 3 gaiac – Research Institute for Ecosystem Analysis and Assessment e.V., RWTH Aachen University, c/o Institute of Environmental Research - Biology V, Worringerweg 1, Aachen D-52056, Germany; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +41-61-267-0480; Fax: +41-61-267-0479. Received: 2 July 2011; in revised form: 25 July 2011 / Accepted: 3 August 2011 / Published: 10 August 2011 Abstract: Butterflies and moths (Lepidoptera) have been suggested for the environmental monitoring of genetically modified (GM) crops due to their suitability as ecological indicators, and because of the possible adverse impact of the cultivation of current transgenic crops. The German Association of Engineers (VDI) has developed guidelines for the standardized monitoring of Lepidoptera describing the use of light traps for adult moths, transect counts for adult butterflies, and visual search for larvae. The guidelines suggest recording adults of Crambid Snout Moths during transect counts in addition to butterflies, and present detailed protocols for the visual search of larvae. -

Insect Pathogens As Biological Control Agents: Back to the Future ⇑ L.A
Journal of Invertebrate Pathology 132 (2015) 1–41 Contents lists available at ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip Insect pathogens as biological control agents: Back to the future ⇑ L.A. Lacey a, , D. Grzywacz b, D.I. Shapiro-Ilan c, R. Frutos d, M. Brownbridge e, M.S. Goettel f a IP Consulting International, Yakima, WA, USA b Agriculture Health and Environment Department, Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK c U.S. Department of Agriculture, Agricultural Research Service, 21 Dunbar Rd., Byron, GA 31008, USA d University of Montpellier 2, UMR 5236 Centre d’Etudes des agents Pathogènes et Biotechnologies pour la Santé (CPBS), UM1-UM2-CNRS, 1919 Route de Mendes, Montpellier, France e Vineland Research and Innovation Centre, 4890 Victoria Avenue North, Box 4000, Vineland Station, Ontario L0R 2E0, Canada f Agriculture and Agri-Food Canada, Lethbridge Research Centre, Lethbridge, Alberta, Canada1 article info abstract Article history: The development and use of entomopathogens as classical, conservation and augmentative biological Received 24 March 2015 control agents have included a number of successes and some setbacks in the past 15 years. In this forum Accepted 17 July 2015 paper we present current information on development, use and future directions of insect-specific Available online 27 July 2015 viruses, bacteria, fungi and nematodes as components of integrated pest management strategies for con- trol of arthropod pests of crops, forests, urban habitats, and insects of medical and veterinary importance. Keywords: Insect pathogenic viruses are a fruitful source of microbial control agents (MCAs), particularly for the con- Microbial control trol of lepidopteran pests. -

Zootaxa, Four New Species of the Genus Chrysoteuchia Hübner
Zootaxa 2485: 33–46 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Four new species of the genus Chrysoteuchia Hübner (Lepidoptera: Crambidae: Crambinae) from China HOUHUN LI & WEICHUN LI College of Life Sciences, Nankai University, Tianjin 300071, China. E-mail: [email protected] Abstract Four new species of the genus Chrysoteuchia Hübner are described from China: C. rotundiprojecta Li & Li, sp. n., C. nonifasciaria Li & Li, sp. n., C. furva Li & Li, sp. n. and C. shafferi Li & Li, sp. n. The female of C. hyalodiscella (Caradja, 1927) is described for the first time. Images of both adults and genitalia of the new species are provided. The distribution data of the thirty-two known Chinese species are presented, along with two maps showing the localities of these species. Key words: Lepidoptera, Crambidae, Crambinae, Chrysoteuchia, new species, China Introduction The genus Chrysoteuchia was established by Hübner in 1825, with Tinea hortuella Hübner, 1796 as the type species. Members of the genus usually have a well-developed sacculus with a distal prong in the male genitalia, which is supposed to be a potential synapomorphy of the genus through our observation. Chrysoteuchia comprises thirty species worldwide (Bleszynski 1965; Inoue 1989; Landry 1995; Chen et al. 2001, 2003), distributed mostly in the Oriental and eastern Palearctic regions except C. culmella (Linnaeus, 1758) which extends to Europe and C. topiaria (Zeller, 1866) which is endemic to North America. Prior to this study, twenty-eight species of Chrysoteuchia were recorded in China. -

Field Crop Pests. the CASE of the HOLEY CORMS: Hey, What's
Section IV: Field Crop Pests. THE CASE OF THE HOLEY CORMS: Hey, What’s Eating Your Timothy Hay M.R. Bush and P. J. Landolt WA State University Extension 2403 S. 18th St., Suite 100, Union Gap, WA 98903-1637 [email protected] & USDA, ARS, YakiMa Agricultural Research Laboratory 5230 Konnowac Pass Road, Wapato, WA 98951 [email protected] During the autuMn of 2014 and 2015, tiMothy hay producers in central Washington reported damage to their fields resulting in large (1/4 acre) patches of poor stands, soMetiMes dead grass, that appeared in the autuMn. Soil saMples and digital iMages of potential perpetrators were brought into WSU Extension offices, but suspect identity could not be confirMed. Evidence indicated that the damage consisted of tiMothy hay corms, overwintering structures, that had been fed on and in soMe cases coMpletely hallowed out. Yield productivity of these fields was marginal at best. A list of potential arthropod suspects was generated. A nuMber of saMpling strategies were employed to trap or sample both the adult and larval stages of these suspects with a focus on lepidoptera and coleoptera. SaMpling strategies included Japanese beetle traps, pitfall traps, sweep net saMpling, and soil sod saMpling on roughly every other week starting in April and continuing through October. In 2016, all fields saMpled were in Ellensburg, WA and most of the fields had reports of poor stands attributed to the perpetrators. The Japanese beetle traps did intercept a wide range of soggy Moths (difficult to identify Microlepidoptera) and pollinators, but few coleoptera froM our list of potential suspects. -

© Амурский Зоологический Журнал. IX(4), 2017. 188-204 © Amurian Zoological Journal. IX(4), 2017. 1
http://www.bgpu.ru/azj/ © Амурский зоологический журнал. IX(4), 2017. 188-204 http://elibrary.ru/title_about.asp?id=30906 © Amurian zoological journal. IX(4), 2017. 188-204 УДК 595.782 ОГНЕВКООБРАЗНЫЕ ЧЕШУЕКРЫЛЫЕ (LEPIDOPTERA: PYRALOIDEA) АМУРСКОЙ ОБЛАСТИ А.Н. Стрельцов A PYRALID MOTHS (LEPIDOPTERA: PYRALOIDEA) OF AMUR REGION A.N. Streltzov Санкт-Петербургский государственный университет, Университетская наб. д. 7-9., Санкт-Петербург, 199034, Россия. E-mail: [email protected] Ключевые слова: огневкообразные чешуекрылые, Lepidoptera, Pyraloidea, фауна, Амурская область Резюме. Первая региональная сводка огневкообразным чешуекрылым (Lepidoptera, Pyraloidea). Для Амурской области указывается 284 вида огневкообразных чешуекрылых, относящиеся к 82 родам из 14 подсемейств 2 семейств. Учитывая степень изученности данного района можно предположить, что предлагаемый список близок к исчерпывающему. Проиводится полная библиография по огневкам региона. Saint Petersburg State University, 7/9 Universitetskaya emb., Saint Petersburg, 199034, Russia. E-mail: streltzov@ mail.ru Key words: pyralid moths, Lepidoptera, Pyraloidea, fauna, Amur region Summary. The first regional report of the pyralid moths (Lepidoptera, Pyraloidea). For the Amur region, 284 species of pyraloid moths are listed, belonging to 82 genera from 14 subfamilies of the 2 families. Given the degree of study of this area, it can be assumed that the proposed list is close to exhaustive. There is a complete bibliography on pyralid moths of this region. Огневкообразные чешуекрылые – Lepidop- тешествие на Амур совершил Р.К. Маак. От tera: Pyraloidea – крупная, широко распро- Нерчинска до устья Паньгухе экспедиция страненная группа бабочек с наибольшим двигалась на плоту, затем на одной из барж разнообразием в тропических широтах. В ус- второго муравьевского сплава проследова- ловиях Дальнего Востока России огневки со- ла до выхода Амура из Хинганского ущелья. -

County Genus Species Species Author Common Name Tribe
County Genus Species Species Author Common Name Tribe Subfamily Family Superfamily Webster County Agonopterix alstroemeriana (Clerck, 1759) Poison Hemlock Moth Depressariini Depressariinae Elachistidae Gelechioidea Webster County Agriphila ruricolellus (Zeller, 1863) Lesser Vagabond Sod Webworm Moth Crambini Crambinae Crambidae Pyraloidea Webster County Alypia octomaculata (Fabricius, 1775) Eight-spotted Forester Moth (None) Agaristinae Noctuidae Noctuoidea Webster County Amorpha juglandis (J E Smith, 1797) Walnut Sphinx Moth Smerinthini Sphinginae Sphingidae Bombycoidea Webster County Amphipyra pyramidoides Guenee, 1852 Copper Underwing Amphipyrini Amphipyrinae Noctuidae Noctuoidea Webster County Anageshna primordialis (Dyar, 1907) Spilomelini Pyraustinae Crambidae Pyraloidea Webster County Anagrapha falcifera (Kirby, 1837) Celery Looper Moth Plusiini Plusiinae Noctuidae Noctuoidea Webster County Apoda biguttata Packard, 1864 Shagreened Slug Moth (None) Limacodinae Limacodidae Zygaenoidea Webster County Argyrotaenia velutinana (Walker, 1863) Red Banded Leafroller Moth Archipini Tortricinae Tortricidae Tortricoidea Webster County Autographa precationis (Guenee, 1852) The Common Looper Moth Plusiini Plusiinae Noctuidae Noctuoidea Webster County Calyptra canadensis (Bethune, 1865) Canadian Owlet Moth Calpini Calpinae Erebidae Noctuoidea Webster County Catocala habilis Grote, 1872 Habilis Underwing Catocalini Erebinae Erebidae Noctuoidea Webster County Ceratomia undulosa (Walker, 1856) Waved Sphinx Moth Sphingini Sphinginae Sphingidae Bombycoidea -

Predator to Prey to Poop: Bats As Microbial Hosts and Insectivorous Hunters
Predator to Prey to Poop: Bats as Microbial Hosts and Insectivorous Hunters A Thesis SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA BY Miranda Galey IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE Dr. Ron Moen, Dr. Jessica R. Sieber September 2020 Copyright © Miranda Galey 2020 Abstract Bat fecal samples are a rich source of ecological data for bat biologists, entomologists, and microbiologists. Feces collected from individual bats can be used to profile the gut microbiome using microbial DNA and to understand bat foraging strategies using arthropod DNA. We used eDNA collected from bat fecal samples to better understand bats as predators in the context of their unique gut physiology. We used high through- put sequencing of the COI gene and 16S rRNA gene to determine the diet composition and gut microbiome composition of three bat species in Minnesota: Eptesicus fuscus, Myotis lucifugus and M. septentrionalis. In our analysis of insect prey, we found that E. fuscus consistently foraged for a higher diversity of beetle species compared to other insects. We found that the proportional frequency of tympanate samples from M. septentrionalis and M. lucifugus was similar, while M. septentrionalis consistently preyed more often upon non-flying species. We used the same set of COI sequences to determine presence of pest species, rare species, and insects not previously observed in Minnesota. We were able to combine precise arthropod identification and the for- aging areas of individually sampled bats to observe possible range expansion of some insects. The taxonomic composition of the bat gut microbiome in all three species was found to be consistent with the composition of a mammalian small intestine. -

1 Appendix 3. Sable Island National Park Taxonomy Report
Appendix 3. Sable Island National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Araneidae Neoscona Neoscona arabesca Clubionidae Clubiona Clubiona abboti Clubiona norvegica Gnaphosidae Micaria Micaria pulicaria Linyphiidae Erigone Erigone aletris Tetragnathidae Pachygnatha Pachygnatha dorothea Thomisidae Xysticus Xysticus ferox Mesostigmata Ascidae Laelapidae Pachylaelapidae Phytoseiidae Opiliones Phalangiidae Phalangium Phalangium opilio Sarcoptiformes Ceratozetidae Trichobates Trichobates incisellus Trichoribates Trombidiformes Bdellidae Erythraeidae Abrolophus Leptus Pygmephoridae Stigmaeidae Tetranychidae Trombidiidae 1 Collembola Entomobryomorpha Entomobryidae Entomobrya Entomobrya multifasciata Lepidocyrtus Lepidocyrtus cyaneus Orchesella Poduromorpha Hypogastruridae Ceratophysella Insecta Lepidoptera Argyresthiidae Argyresthia Argyresthia annettella Autostichidae Glyphidocera Glyphidocera septentrionella Choreutidae Tebenna Tebenna onustana Coleophoridae Coleophora Coleophora cornella Coleophora duplicis group Coleophora glaucicolella Coleophora granifera Coleophora mayrella Coleophora quadrilineella Coleophora rosacella Crambidae Agriphila Agriphila ruricolellus Agriphila vulgivagellus Chrysoteuchia Chrysoteuchia topiarius Crambus Crambus leachellus Crambus perlella Fissicrambus Pediasia Pediasia trisecta Scoparia Scoparia biplagialis Depressariidae Depressaria Depressaria alienella Elachistidae Elachista 2 Erebidae Orgyia Orgyia leucostigma Spilosoma Spilosoma -

Crambidae: Lepidoptera) of Ohio: Characterization, Host Associations and Revised Species Accounts
Crambinae (Crambidae: Lepidoptera) of Ohio: Characterization, Host Associations and Revised Species Accounts THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Devon A Rogers Graduate Program in Entomology The Ohio State University 2014 Master's Examination Committee: Dr. David J. Shetlar - Advisor Dr. Steve Passoa Dr. Andy Michel Dr. Dave Gardiner Copyright by Devon Ashley Rogers 2014 Abstract A review of the North American Crambinae sod webworm taxonomy, phylogenetic history, and biology is presented. Traditional analysis, combined with modern genetic analysis has changed and solidified the placement of these species. Previously cryptic and unidentifiable larvae were identified using genetic analysis of the mitochondrial CO1 gene and an evaluation of potential host plant associations is given. DNA sequencing is a useful tool that can be used to identify unknown sod webworm larvae, including the especially difficult to identify first and second instar larvae. Only Parapediasia teterrella larvae were recovered from the short-cut, golf course-type, creeping bentgrass (Agrostis stolonifera), as was a single Agriphila ruricolella. Fissicrambus mutabilis was obtained from lawn-height Kentucky bluegrass (Poa pratensis) and turf type tall fescue (Festuca arundinacea). Sod webworm adults were monitored with a standard blacklight trap between 2009 and 2013. Each year 14 species were recovered from the light trap. Species obtained from the managed turfgrass yielded only a fraction of the number of species attracted to the light trap. The sod webworm species Euchromius ocellus first appeared in late 2012. This is a first report for this species in Ohio. -

Cranberry Girdler; Sod Webworm Chrysoteuchia Topiaria
44 MAJOR INSECTS: Cranberry Girdler CHAPTER TWENTY Cranberry Girdler; Sod Webworm Chrysoteuchia topiaria David L. Overhulser Insect and hosts The larval stages of the cranberry girdler commonly feed on the crowns and roots of grasses. Cran- berry girdler feeding damage to the root-collar area of conifer seedlings has been reported in Oregon, Wash- ington, and Idaho. Two-year-old Douglas-fir, noble fir, larch, and spruce stock are frequently dam- aged. Other stock types occasionally damaged are 1+0 and 3+0 Douglas-fir and 1+0 larch. Cranberry girdler damage may be confused with: Lower stem canker Mechanical damage Rodent feeding Root weevil damage Figure 20-1. Cranberry girdler damage to 2+0 noble fir seedlings. Oregon Department of Forestry photo. Symptoms jerky movements for short distances. of the effects of predation and In late summer and early fall, Female moths deposit eggs on and disease on overwintering larvae. cranberry girdler larvae eat patches around nursery stock. Eggs hatch in of bark and cortex from the root 3 to 5 days. Larvae feed in nursery collars and roots of seedlings beds from June to October. The Loss potential (Figures 20-1 and 20-2). Damage is feeding of late-instar larvae from Nurseries next to grass fields, detected when stock is lifted and August to October causes the most graded or when severely damaged which are prime cranberry girdler damage to seedlings. In late fall, habitat, are most vulnerable. When seedlings still in the beds turn yellow larvae spin a cocoon in the soil in the fall. the moth population is high, 15 to 20 where they overwinter (Figure 20-4).