Phenylmercuric Salts 1657 Ingrowing Toenails
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rpt POL-TOXIC AIR POLLUTANTS 98 BY
SWCAA TOXIC AIR POLLUTANTS '98 by CAS ASIL TAP SQER CAS No HAP POLLUTANT NAME HAP CAT 24hr ug/m3 Ann ug/m3 Class lbs/yr lbs/hr none17 BN 1750 0.20 ALUMINUM compounds none0.00023 AY None None ARSENIC compounds (E649418) ARSENIC COMPOUNDS none0.12 AY 20 None BENZENE, TOLUENE, ETHYLBENZENE, XYLENES BENZENE none0.12 AY 20 None BTEX BENZENE none0.000083 AY None None CHROMIUM (VI) compounds CHROMIUM COMPOUN none0.000083 AY None None CHROMIUM compounds (E649962) CHROMIUM COMPOUN none0.0016 AY 0.5 None COKE OVEN COMPOUNDS (E649830) - CAA 112B COKE OVEN EMISSIONS none3.3 BN 175 0.02 COPPER compounds none0.67 BN 175 0.02 COTTON DUST (raw) none17 BY 1,750 0.20 CYANIDE compounds CYANIDE COMPOUNDS none33 BN 5,250 0.60 FIBROUS GLASS DUST none33 BY 5,250 0.60 FINE MINERAL FIBERS FINE MINERAL FIBERS none8.3 BN 175 0.20 FLUORIDES, as F, containing fluoride, NOS none0.00000003 AY None None FURANS, NITRO- DIOXINS/FURANS none5900 BY 43,748 5.0 HEXANE, other isomers none3.3 BN 175 0.02 IRON SALTS, soluble as Fe none00 AN None None ISOPROPYL OILS none0.5 AY None None LEAD compounds (E650002) LEAD COMPOUNDS none0.4 BY 175 0.02 MANGANESE compounds (E650010) MANGANESE COMPOU none0.33 BY 175 0.02 MERCURY compounds (E650028) MERCURY COMPOUND none33 BY 5,250 0.60 MINERAL FIBERS ((fine), incl glass, glass wool, rock wool, slag w FINE MINERAL FIBERS none0.0021 AY 0.5 None NICKEL 59 (NY059280) NICKEL COMPOUNDS none0.0021 AY 0.5 None NICKEL compounds (E650036) NICKEL COMPOUNDS none0.00000003 AY None None NITROFURANS (nitrofurans furazolidone) DIOXINS/FURANS none0.0013 -

FOI 161-112 Document 1
\/ Fw: IDC - Mercury L fy [SEC=:UNCLASSIFIED] 09/12/2010 04:23 PM - This is the fi rst of two emails I will send - this is our response to the questions posed by the UNEP f""I secretariat after the first in June. INC ...., C Kind regards, QJ E :::l ..*******��***************�************ u o International Coordination Team c Office of Parliamentary and Strategic Support Therapeutic Goods Administration E-mail: [email protected] ----- Forwarded n 09/12/20"10 04:21 PM ---_. To 'ilenvironmentgov,au> · cc !ilenvironment.gov,au>, environment.gov,au>_ "" 11/11/2010 .. .. 02:14 PM Subject Re: IDC - Mercury LBI- Friday 23 July [SEC=UNCLASSIFIED} Dear_ Please find attached TGA's contribution to the mercury data gaps identified by the UNEP Secretariat. Should you have any queries or concerns relating to tllis information, we would be very happy to discuss these at our meeting tomorrow (Friday 12 November at 3pm), TGA participants at tomorrow's meeting will be: Devices Authorisation Office of Scientific Evaluation Services _ill also be there, representing the International Coordination team. Please note that we are correctly identified as stakeholders for the Regulation sector (arrangements for labelling products containing mercury), but mistakenly included as a stakeholder for the sector for uni ntentional emission (biomedical waste i Ilcineration), Kind regards, International Coordination Tearn Office of Parliamentary aild Strategic Support Therapeutic Goods Administration E�mail: [email protected] o - Mercury in ther�peutic products - Response for UNEP INC Oct 201 O.DOCX Dear Colleagues FOl owlng u� ercury �BI meeting on FridLY'23 July, we would li ke to t from t� �C � M request for )tell assl�� ce In addressing the mercury data gaps that the UNEP Secretariat has identified. -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Assessment of the Antibiotic Resistance Effects of Biocides
Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR Assessment of the Antibiotic Resistance Effects of Biocides The SCENIHR adopted this opinion at the 28th plenary on 19 January 2009 after public consultation. 1 Antibiotic Resistance Effects of Biocides About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Products (SCCP), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR), and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMEA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCENIHR Questions concerning emerging or newly-identified risks and on broad, complex or multi- disciplinary issues requiring a comprehensive assessment of risks to consumer safety or public health and related issues not covered by other Community risk-assessment bodies. In particular, the Committee addresses questions related to potential risks associated with interaction of risk factors, synergic effects, cumulative effects, antimicrobial resistance, new technologies such as nanotechnologies, medical devices, tissue engineering, blood products, fertility reduction, cancer of endocrine organs, physical hazards such as noise and electromagnetic fields and methodologies for assessing new risks. -

Mercury Contamination in Man and His Environment
TECHNICAL REPORTS SERIES No. 137 Mercury Contamination in Man and his Environment A JOINT UNDERTAKING BY THE INTERNATIONAL LABOUR ORGANISATION. THE FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS. THE WORLD HEALTH ORGANIZATION AND THE INTERNATIONAL ATOMIC ENERGY AGENCY J WJ INTERNATIONAL ATOMIC ENERGY AGENCY, VIENNA, 1972 MERCURY CONTAMINATION IN MAN AND HIS ENVIRONMENT TECHNICAL REPORTS SERIES No. 137 MERCURY CONTAMINATION IN MAN AND HIS ENVIRONMENT A JOINT UNDERTAKING BY THE INTERNATIONAL LABOUR ORGANISATION, THE FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS, THE WORLD HEALTH ORGANIZATION AND THE INTERNATIONAL ATOMIC ENERGY AGENCY INTERNATIONAL ATOMIC ENERGY AGENCY VIENNA, 1972 MERCURY CONTAMINATION IN MAN AND HIS ENVIRONMENT IAEA, VIENNA, 1972 STI/DOC/lO/137 Printed by the IAEA in Austria July 1972 FOREWORD In May 1967, at a Symposium organized by the International Atomic Energy Agency in Amsterdam, the special problems of food and environ- mental contamination by mercury were discussed by world experts on the subject and by representatives of FAO, WHO and IAEA. One of the recom- mendations made by this meeting was that the international organizations of the United Nations family should assist in the collection and distribution of information on environmental mercury. Subsequently, the organizations concerned agreed that a handbook on mercury contamination would be es- pecially useful. This would deal with sources of mercury in relation to man and his environment; with physical and biological transfer processes that determine its distribution; with analytical methods for determining mercury and its compounds as environmental contaminants; with actual concentrations of mercury found in the environment, in living organisms and in man; and with its toxicology in animals and man. -

Heavy-Atom Kinetic Isotope Effects
A UNITED STATES DEPARTMENT OF COMMERCE NATL PUBLICATION NBS SPECIAL PUBLICATION 349 •Nr., of** Heavy-Atom Kinetic Isotope Effects An Indexed Bibliography U.S. EPARTMENT OF COMMERCE National Bureau of jQ^^^tandards 00 SI — NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—^Linac -

Chemical Safety and Waste Management Manual
Chemical Safety and Waste Management Manual University of Alabama at Birmingham Department of Occupational Health & Safety Chemical Safety Division 2002 EDITION 1. INTRODUCTION In a comparatively short time, the University of Alabama at Birmingham has gained significant recognition as a center of excellence for teaching, medical services and research programs. This is a highly commendable achievement and one that could not have been realized without the continued support and dedication of faculty, staff members, and employees. Similar unfailing cooperation and support are necessary for the institution to be equally successful in its development of a comprehensive occupational health and safety program for the protection of University personnel, students, and the surrounding community. An important part of this program is concerned with the safe and prudent handling of chemicals and their proper legal disposal as regulated by the Environmental Protection Agency (EPA) and the Alabama Department of Environmental Management (ADEM). Almost every laboratory and many allied and support personnel at UAB use chemicals in their daily activities. It is the purpose of this manual to describe the operation of the Chemical Safety Program and to provide guidance in establishing safe work practices for the use of chemicals. This program applies to all work operations at this University where employees may be exposed to hazardous substances under normal working conditions or during an emergency. The Chemical Safety and Waste Management Manual combines both the Chemical Hygiene Plan for laboratories and the Hazard Communication Program for maintenance, environmental services, and other support personnel. The Occupational Safety and Health Administration (OSHA) Hazard Communication Standard may be found at : http://www.osha- slc.gov/OshStd_data/1910_1200.html. -

Act on Preventing Environmental Pollution of Mercury” (About Mercury-Added Products for New Use)
June 2015 Ministerial Ordinance under “Act on Preventing Environmental Pollution of Mercury” (about mercury-added products for new use) Chemical Management Policy Division, Manufacturing Industries Bureau, Ministry of Economy, Trade and Industry, Japan Environmental Health and Safety Division, Environmental Health Department, Environmental Policy Bureau, Ministry of the Environment, Japan 1. Background of enactment of the ministerial ordinance Act on Preventing Environmental Pollution of Mercury (Act No. 42 of 2015), as a measure to ensure the implementation of Article 4(6) of the Minamata Convention on Mercury (hereinafter referred to as “the Convention”), lays out the basic principle of banning the manufacture and distribution in commerce of mercury-added products not covered by mercury-added products for known use (hereinafter referred to as “mercury added products for new use”) unless the use of such products demonstrates environmental or human health benefits. Further, businesses intending to be engaged in the manufacture or distribution in commerce of “mercury-added products for new use” are required to carry out a self-assessment of use of their “mercury-added products for new use” and notify its result to the competent minister. The Ministerial Ordinance of the Act specifies mercury-added products for known use and the method of the self-assessment of the manufacture or distribution in commerce of “mercury-added products for new use”. 2. Outline (1) Mercury-added products for known use “Mercury-added products for known use” are listed in Annex. However, based on identification of current practice of use of “mercury-added products for known use”, addition or revision to Annex may occur in the future. -
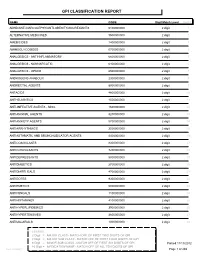
Gpi Drug Classification Report
GPI CLASSIFICATION REPORT NAME CODE Digit Match Level Count ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS 6100000000 2 digit 1 ALTERNATIVE MEDICINES 9500000000 2 digit 2 AMEBICIDES 1400000000 2 digit 3 AMINOGLYCOSIDES 0700000000 2 digit 4 ANALGESICS - ANTI-INFLAMMATORY 6600000000 2 digit 5 ANALGESICS - NONNARCOTIC 6400000000 2 digit 6 ANALGESICS - OPIOID 6500000000 2 digit 7 ANDROGENS-ANABOLIC 2300000000 2 digit 8 ANORECTAL AGENTS 8900000000 2 digit 9 ANTACIDS 4800000000 2 digit 10 ANTHELMINTICS 1500000000 2 digit 11 ANTI-INFECTIVE AGENTS - MISC. 1600000000 2 digit 12 ANTIANGINAL AGENTS 3200000000 2 digit 13 ANTIANXIETY AGENTS 5700000000 2 digit 14 ANTIARRHYTHMICS 3500000000 2 digit 15 ANTIASTHMATIC AND BRONCHODILATOR AGENTS 4400000000 2 digit 16 ANTICOAGULANTS 8300000000 2 digit 17 ANTICONVULSANTS 7200000000 2 digit 18 ANTIDEPRESSANTS 5800000000 2 digit 19 ANTIDIABETICS 2700000000 2 digit 20 ANTIDIARRHEALS 4700000000 2 digit 21 ANTIDOTES 9300000000 2 digit 22 ANTIEMETICS 5000000000 2 digit 23 ANTIFUNGALS 1100000000 2 digit 24 ANTIHISTAMINES 4100000000 2 digit 25 ANTIHYPERLIPIDEMICS 3900000000 2 digit 26 ANTIHYPERTENSIVES 3600000000 2 digit 27 ANTIMALARIALS 1300000000 2 digit 28 LEGEND: 2 Digit = MAJOR CLASS - MATCH OFF OF FIRST TWO DIGITS OF GPI 4 Digit = MAJOR SUB CLASS - MATCH OFF OF FIRST FOUR DIGITS OF GPI 6 Digit = MINOR SUB CLASS - MATCH OFF OF FIRST SIX DIGITS OF GPI Printed:11/13/2012 10 Digit = MEDICATION NAME - MATCH OFF OF ALL TEN DIGITS OF GPI Lee Cooper Page 1 of 265 NAME CODE Digit Match Level Count ANTIMYASTHENIC AGENTS -

21 CFR Ch. I (4–1–19 Edition) § 310.545
§ 310.545 21 CFR Ch. I (4–1–19 Edition) for marketing. In the absence of an ap- Chloroxylenol proved new drug application or abbre- Cloxyquin viated new drug application, such prod- Coal tar uct is also misbranded under section Dibenzothiophene Estrone 502 of the act. Magnesium aluminum silicate (c) Clinical investigations designed Magnesium sulfate to obtain evidence that any drug prod- Phenol uct labeled, represented, or promoted Phenolate sodium for OTC use as a smoking deterrent is Phenyl salicylate safe and effective for the purpose in- Povidone-iodine tended must comply with the require- Pyrilamine maleate ments and procedures governing the Resorcinol (as single ingredient) use of investigational new drugs set Resorcinol monoacetate (as single ingre- dient) forth in part 312 of this chapter. Salicylic acid (over 2 up to 5 percent) (d) After May 7, 1991, any such OTC Sodium borate drug product containing cloves, cori- Sodium thiosulfate ander, eucalyptus oil, ginger (Ja- Tetracaine hydrochloride maica), lemon oil (terpeneless), licorice Thymol root extract, menthol, methyl salicy- Vitamin E late, quinine ascorbate, silver nitrate, Zinc oxide and/or thymol initially introduced or Zinc stearate initially delivered for introduction into Zinc sulfide interstate commerce that is not in (2) Anticaries drug products—(i) Ap- compliance with this section is subject proved as of May 7, 1991. to regulatory action. After December 1, 1993, any such OTC drug product con- Hydrogen fluoride Sodium carbonate taining lobeline (in the form of lobeline Sodium monofluorophosphate (6 percent sulfate or natural lobelia alkaloids or rinse) Lobelia inflata herb), povidone-silver ni- Sodium phosphate trate, silver acetate, or any other in- gredients initially introduced or ini- (ii) Approved as of October 7, 1996. -

Topical Drugs 35 Francisco M
35_623_652 05.11.2005 11:22 Uhr Seite 623 Chapter 35 Topical Drugs 35 Francisco M. Brandão, An Goossens, Antonella Tosti Contents medication habits. Prescribing habits are changing, 35.1 Incidence and Prevalence . 623 and some medicaments that were common allergens 20–30 years ago,such as sulfonamides,penicillin,and 35.2 Factors Predisposing antihistamines, have now been replaced by other al- to Medicament Contact Dermatitis . 624 lergenic drugs, such as nonsteroidal anti-inflamma- 35.3 Clinical Patterns of Contact Reactions . 624 tory drugs (NSAID), and corticosteroids. 35.3.1 Irritant Contact Dermatitis . 624 The real incidence of adverse reactions to topical 35.3.2 Contact Urticaria . 625 medicaments is not known, and most of the data 35.3.3 Other Important Clinical Patterns . 625 about prevalence are quite old. Bandmann et al. [1] 35.3.4 Allergic Contact Dermatitis . 626 found that 14% of 4,000 patients tested in several Eu- 35.4 Allergens . 627 ropean countries were allergic to medicaments.Blon- 35.4.1 Local Anesthetics . 627 deel et al. [2] found a much higher incidence – 54.6%. 35.4.2 Antibiotics and Antimicrobials . 627 Still, in Belgium [3], 17% of 2,025 patients were aller- 35.4.3 Antivirals . 629 gic to the ingredients of pharmaceutical products, 35.4.4 Antimycotics . 629 while in Italy, in the 1980s [4], about 20.5% of 8,230 35.4.5 Corticosteroids . 630 patients were allergic to topical drugs. In Sweden, 35.4.6 Antihistamines . 632 40% of all recorded allergic reactions were due to 35.4.7 Nonsteroidal Anti-Inflammatory Drugs . -

Mercury Waste Solutions
Exhibit B: MERCURY WASTE SOLUTIONS ACCEPTANCE GUIDELINES This information is being provided to assist you in determining which mercury-bearing materials are acceptable for processing and mercury recovery by Mercury Waste Solutions, Inc. (MWSI). Materials and compounds accepted for processing may be subject to regulations as hazardous wastes. Other non-mercury materials are also accepted for recycling. I. Mercury Waste Solutions is permitted to accept the following materials: A. Acceptable Mercury Wastes and Codes D001 as an oxidizer, D002, D004-D011, U151, K071, K106 & P092 Universal Waste batteries, lamps and thermostats B. Other Acceptable Materials Lighting Ballasts (PCB and non-PCB) Batteries (alkaline, lithium, and nickel cadmium) Obsolete Computer Equipment II. Mercury Waste Solutions is NOT permitted to accept the following materials: w Any F, K, P or U-listed wastes (other than U151 and K106) w Radioactive materials w Reactive materials (D003) (lithium batteries are accepted under Universal Waste Rule) w Regulated medical wastes w Dioxins w Pesticides and herbicides MERCURY WASTE SOLUTIONS ACCEPTABLE MATERIALS ACTIVATED CARBON/CHARCOAL BATTERIES Alkaline (with/without mercury - Universal Waste) A to N Batteries (Coast Guard, gel cell batteries) Lithium (Universal Waste) Mercuric Oxide (Universal Waste) Nickel Cadmium (Universal Waste) Zinc Air (with/ without mercury - Universal Waste) Zinc Carbon (with/without mercury - Universal Waste) CHLOR ALKALI PRODUCTION DEBRIS METALLIC MERCURY (pure/impure) CHLOR ALKALI PRODUCTION RESIDUE