Articles and Also and Instrumental Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Image – Action – Space
Image – Action – Space IMAGE – ACTION – SPACE SITUATING THE SCREEN IN VISUAL PRACTICE Luisa Feiersinger, Kathrin Friedrich, Moritz Queisner (Eds.) This publication was made possible by the Image Knowledge Gestaltung. An Interdisciplinary Laboratory Cluster of Excellence at the Humboldt-Universität zu Berlin (EXC 1027/1) with financial support from the German Research Foundation as part of the Excellence Initiative. The editors like to thank Sarah Scheidmantel, Paul Schulmeister, Lisa Weber as well as Jacob Watson, Roisin Cronin and Stefan Ernsting (Translabor GbR) for their help in editing and proofreading the texts. This work is licensed under a Creative Commons Attribution-NonCommercial-No-Derivatives 4.0 License. For details go to https://creativecommons.org/licenses/by-nc-nd/4.0/. Copyrights for figures have been acknowledged according to best knowledge and ability. In case of legal claims please contact the editors. ISBN 978-3-11-046366-8 e-ISBN (PDF) 978-3-11-046497-9 e-ISBN (EPUB) 978-3-11-046377-4 Library of Congress Control Number: 2018956404 Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche National bibliothek lists this publication in the Deutsche Nationalbibliographie; detailed bibliographic data are available on the internet at http://dnb.dnb.de. © 2018 Luisa Feiersinger, Kathrin Friedrich, Moritz Queisner, published by Walter de Gruyter GmbH, Berlin/Boston The book is published with open access at www.degruyter.com, https://www.doabooks.org and https://www.oapen.org. Cover illustration: Malte Euler Typesetting and design: Andreas Eberlein, aromaBerlin Printing and binding: Beltz Bad Langensalza GmbH, Bad Langensalza Printed in Germany www.degruyter.com Inhalt 7 Editorial 115 Nina Franz and Moritz Queisner Image – Action – Space. -

Glossary Glossary
Glossary Glossary Albedo A measure of an object’s reflectivity. A pure white reflecting surface has an albedo of 1.0 (100%). A pitch-black, nonreflecting surface has an albedo of 0.0. The Moon is a fairly dark object with a combined albedo of 0.07 (reflecting 7% of the sunlight that falls upon it). The albedo range of the lunar maria is between 0.05 and 0.08. The brighter highlands have an albedo range from 0.09 to 0.15. Anorthosite Rocks rich in the mineral feldspar, making up much of the Moon’s bright highland regions. Aperture The diameter of a telescope’s objective lens or primary mirror. Apogee The point in the Moon’s orbit where it is furthest from the Earth. At apogee, the Moon can reach a maximum distance of 406,700 km from the Earth. Apollo The manned lunar program of the United States. Between July 1969 and December 1972, six Apollo missions landed on the Moon, allowing a total of 12 astronauts to explore its surface. Asteroid A minor planet. A large solid body of rock in orbit around the Sun. Banded crater A crater that displays dusky linear tracts on its inner walls and/or floor. 250 Basalt A dark, fine-grained volcanic rock, low in silicon, with a low viscosity. Basaltic material fills many of the Moon’s major basins, especially on the near side. Glossary Basin A very large circular impact structure (usually comprising multiple concentric rings) that usually displays some degree of flooding with lava. The largest and most conspicuous lava- flooded basins on the Moon are found on the near side, and most are filled to their outer edges with mare basalts. -

A Review of Experimental Techniques for Aerosol Hygroscopicity Studies
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2019-398 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 3 May 2019 c Author(s) 2019. CC BY 4.0 License. 1 A review of experimental techniques for aerosol hygroscopicity studies 2 3 Mingjin Tang,1,* Chak K Chan,2,* Yong Jie Li,3 Hang Su,4,5 Qingxin Ma,6 Zhijun Wu,7 Guohua 4 Zhang,1 Zhe Wang,8 Maofa Ge,9 Min Hu,7 Hong He,6,10,11 Xinming Wang1,10,11 5 6 1 State Key Laboratory of Organic Geochemistry and Guangdong Key Laboratory of 7 Environmental Protection and Resources Utilization, Guangzhou Institute of Geochemistry, 8 Chinese Academy of Sciences, Guangzhou 510640, China 9 2 School of Energy and Environment, City University of Hong Kong, Kowloon, Hong Kong, 10 China 11 3 Department of Civil and Environmental Engineering, Faculty of Science and Technology, 12 University of Macau, Avenida da Universidade, Taipa, Macau, China 13 4 Center for Air Pollution and Climate Change Research (APCC), Institute for Environmental 14 and Climate Research (ECI), Jinan University, Guangzhou 511443, China 15 5 Department of Multiphase Chemistry, Max Planck Institute for Chemistry, Mainz 55118, 16 Germany 17 6 State Key Joint Laboratory of Environment Simulation and Pollution Control, Research 18 Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China 19 7 State Key Joint Laboratory of Environmental Simulation and Pollution Control, College of 20 Environmental Sciences and Engineering, Peking University, Beijing 100871, China 21 8 Department of Civil and Environmental Engineering, The Hong Kong Polytechnic University, 22 Hong Kong, China 23 9 State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of 24 Chemistry, Chinese Academy of Sciences, Beijing 100190, China 25 10 University of Chinese Academy of Sciences, Beijing 100049, China 1 Atmos. -

Byzantium and France: the Twelfth Century Renaissance and the Birth of the Medieval Romance
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-1992 Byzantium and France: the Twelfth Century Renaissance and the Birth of the Medieval Romance Leon Stratikis University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Modern Languages Commons Recommended Citation Stratikis, Leon, "Byzantium and France: the Twelfth Century Renaissance and the Birth of the Medieval Romance. " PhD diss., University of Tennessee, 1992. https://trace.tennessee.edu/utk_graddiss/2521 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Leon Stratikis entitled "Byzantium and France: the Twelfth Century Renaissance and the Birth of the Medieval Romance." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Modern Foreign Languages. Paul Barrette, Major Professor We have read this dissertation and recommend its acceptance: James E. Shelton, Patrick Brady, Bryant Creel, Thomas Heffernan Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a dissertation by Leon Stratikis entitled Byzantium and France: the Twelfth Century Renaissance and the Birth of the Medieval Romance. -

Glossary of Lunar Terminology
Glossary of Lunar Terminology albedo A measure of the reflectivity of the Moon's gabbro A coarse crystalline rock, often found in the visible surface. The Moon's albedo averages 0.07, which lunar highlands, containing plagioclase and pyroxene. means that its surface reflects, on average, 7% of the Anorthositic gabbros contain 65-78% calcium feldspar. light falling on it. gardening The process by which the Moon's surface is anorthosite A coarse-grained rock, largely composed of mixed with deeper layers, mainly as a result of meteor calcium feldspar, common on the Moon. itic bombardment. basalt A type of fine-grained volcanic rock containing ghost crater (ruined crater) The faint outline that remains the minerals pyroxene and plagioclase (calcium of a lunar crater that has been largely erased by some feldspar). Mare basalts are rich in iron and titanium, later action, usually lava flooding. while highland basalts are high in aluminum. glacis A gently sloping bank; an old term for the outer breccia A rock composed of a matrix oflarger, angular slope of a crater's walls. stony fragments and a finer, binding component. graben A sunken area between faults. caldera A type of volcanic crater formed primarily by a highlands The Moon's lighter-colored regions, which sinking of its floor rather than by the ejection of lava. are higher than their surroundings and thus not central peak A mountainous landform at or near the covered by dark lavas. Most highland features are the center of certain lunar craters, possibly formed by an rims or central peaks of impact sites. -

Understanding Second-Person Storytelling
Evgenia Iliopoulou Because of You: Understanding Second-Person Storytelling Lettre Evgenia Iliopoulou born in 1986, lives in Zurich, Switzerland, and specializes in narratology, theory of literature and interdisciplinary approaches within Comparative Literature. She holds an undergraduate degree in Greek Philology from her hometown University of Patras, Greece, and an MA in Comparative Literature from Ludwig Maximilian University of Munich, Germany. In 2014, during her doctoral studies, Zurich University sponsored her participation in the summer session of School of Theory and Criticism at Cornell University in the US. Evgenia Iliopoulou Because of You: Understanding Second-Person Storytelling This work was accepted as a PhD thesis by the Faculty of Arts and Social Scien- ces, University of Zurich in the fall semester 2017 on the recommendation of the Doctoral Committee: Prof. Dr. Thomas Fries «main supervisor», Prof. Dr. Sandro Zanetti. Published with the support of the Swiss National Science Foundation. Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Na- tionalbibliografie; detailed bibliographic data are available in the Internet at http://dnb.d-nb.de This work is licensed under the Creative Commons Attribution-NonCommercial-No- Derivatives 4.0 (BY-NC-ND) which means that the text may be used for non-commer- cial purposes, provided credit is given to the author. For details go to http://creativecommons.org/licenses/by-nc-nd/4.0/ To create an adaptation, translation, or derivative of the original work and for commer- cial use, further permission is required and can be obtained by contacting rights@ transcript-verlag.de Creative Commons license terms for re-use do not apply to any content (such as graphs, figures, photos, excerpts, etc.) not original to the Open Access publication and further permission may be required from the rights holder. -

A Complete Bibliography of Publications in Journal for the History of Astronomy
A Complete Bibliography of Publications in Journal for the History of Astronomy Nelson H. F. Beebe University of Utah Department of Mathematics, 110 LCB 155 S 1400 E RM 233 Salt Lake City, UT 84112-0090 USA Tel: +1 801 581 5254 FAX: +1 801 581 4148 E-mail: [email protected], [email protected], [email protected] (Internet) WWW URL: http://www.math.utah.edu/~beebe/ 10 May 2021 Version 1.28 Title word cross-reference $8.95 [Had84]. $87 [CWW17]. $89.95 [Gan15]. 8 = 1;2;3 [Cov15]. $90 [Ano15f]. $95 [Swe17c]. c [Kin87, NRKN16, Rag05]. mul [Kur19]. muld [Kur19]. [Kur19]. · $100 [Apt14]. $120 [Hen15b]. $127 [Llo15]. 3 [Ano15f, Ash82, Mal10, Ste12a]. ∆ [MS04]. $135.00 [Smi96]. $14 [Sch15]. $140 [GG14]. $15 [Jar90]. $17.95 [Had84]. $19.95 -1000 [Hub83]. -4000 [Gin91b]. -601 [Mul83, Nau98]. 20 [Ost07]. 2000 [Eva09b]. [Hub83]. -86 [Mar75]. -Ft [Edd71b, Mau13]. $24.95 [Lep14, Bro90]. $27 [W lo15]. $29 -inch [Ost07]. -year [GB95]. -Year-Old [Sha14]. $29.25 [Hea15]. $29.95 [Eva09b]. [Ger17, Mes15]. 30 [Mau13]. $31.00 [Bra15]. $34 [Sul14]. $35 Zaga´_ n [CM10]. [Ano15f, Dev14b, Lau14, Mir17, Rap15a]. $38 [Dan19b, Vet19]. $39.95 [Mol14b]. /Catalogue [Kun91]. /Charles [Tur07]. $39.99 [Bec15]. $40 [Dun20, LF15, Rob86]. /Collected [Gin93]. /Heretic [Tes10]. 40 [Edd71b]. $42 [Nau98]. $45 [Kes15]. /the [War08]. $49.95 [Ree20]. $49.99 [Bec15]. $50 [Kru17, Rem15]. $55 [Bon19b]. $60 [Mal15]. 0 [Ave18, Hei14a, Oes15, Swe17c, Wlo15, 600 [GB95]. $72 [Ave18]. $79 [Wil15]. 1 2 Ash82]. [Dic97a]. 1970 [Kru08]. 1971 [Wil75]. 1973 [Doe19]. 1975 [Ost80]. 1976 [Gin02d]. 1979 1 [Ano15f, BH73a, Ber14, Bru78b, Eva87b, [Ano78d]. -

Constraining the Evolutionary History of the Moon and the Inner Solar System: a Case for New Returned Lunar Samples
Space Sci Rev (2019) 215:54 https://doi.org/10.1007/s11214-019-0622-x Constraining the Evolutionary History of the Moon and the Inner Solar System: A Case for New Returned Lunar Samples Romain Tartèse1 · Mahesh Anand2,3 · Jérôme Gattacceca4 · Katherine H. Joy1 · James I. Mortimer2 · John F. Pernet-Fisher1 · Sara Russell3 · Joshua F. Snape5 · Benjamin P. Weiss6 Received: 23 August 2019 / Accepted: 25 November 2019 / Published online: 2 December 2019 © The Author(s) 2019 Abstract The Moon is the only planetary body other than the Earth for which samples have been collected in situ by humans and robotic missions and returned to Earth. Scien- tific investigations of the first lunar samples returned by the Apollo 11 astronauts 50 years ago transformed the way we think most planetary bodies form and evolve. Identification of anorthositic clasts in Apollo 11 samples led to the formulation of the magma ocean concept, and by extension the idea that the Moon experienced large-scale melting and differentiation. This concept of magma oceans would soon be applied to other terrestrial planets and large asteroidal bodies. Dating of basaltic fragments returned from the Moon also showed that a relatively small planetary body could sustain volcanic activity for more than a billion years after its formation. Finally, studies of the lunar regolith showed that in addition to contain- ing a treasure trove of the Moon’s history, it also provided us with a rich archive of the past 4.5 billion years of evolution of the inner Solar System. Further investigations of samples returned from the Moon over the past five decades led to many additional discoveries, but also raised new and fundamental questions that are difficult to address with currently avail- able samples, such as those related to the age of the Moon, duration of lunar volcanism, the Role of Sample Return in Addressing Major Questions in Planetary Sciences Edited by Mahesh Anand, Sara Russell, Yangting Lin, Meenakshi Wadhwa, Kuljeet Kaur Marhas and Shogo Tachibana B R. -

Modeling and Mapping of the Structural Deformation of Large Impact Craters on the Moon and Mercury
MODELING AND MAPPING OF THE STRUCTURAL DEFORMATION OF LARGE IMPACT CRATERS ON THE MOON AND MERCURY by JEFFREY A. BALCERSKI Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Earth, Environmental, and Planetary Sciences CASE WESTERN RESERVE UNIVERSITY August, 2015 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Jeffrey A. Balcerski candidate for the degree of Doctor of Philosophy Committee Chair Steven A. Hauck, II James A. Van Orman Ralph P. Harvey Xiong Yu June 1, 2015 *we also certify that written approval has been obtained for any proprietary material contained therein ~ i ~ Dedicated to Marie, for her love, strength, and faith ~ ii ~ Table of Contents 1. Introduction ............................................................................................................1 2. Tilted Crater Floors as Records of Mercury’s Surface Deformation .....................4 2.1 Introduction ..............................................................................................5 2.2 Craters and Global Tilt Meters ................................................................8 2.3 Measurement Process...............................................................................12 2.3.1 Visual Pre-selection of Candidate Craters ................................13 2.3.2 Inspection and Inclusion/Exclusion of Altimetric Profiles .......14 2.3.3 Trend Fitting of Crater Floor Topography ................................16 2.4 Northern -

Ancient Lamps in the J. Paul Getty Museum
ANCIENT LAMPS THE J. PAUL GETTY MUSEUM Ancient Lamps in the J. Paul Getty Museum presents over six hundred lamps made in production centers that were active across the ancient Mediterranean world between 800 B.C. and A.D. 800. Notable for their marvelous variety—from simple clay saucers GETTYIN THE PAUL J. MUSEUM that held just oil and a wick to elaborate figural lighting fixtures in bronze and precious metals— the Getty lamps display a number of unprecedented shapes and decors. Most were made in Roman workshops, which met the ubiquitous need for portable illumination in residences, public spaces, religious sanctuaries, and graves. The omnipresent oil lamp is a font of popular imagery, illustrating myths, nature, and the activities and entertainments of daily life in antiquity. Presenting a largely unpublished collection, this extensive catalogue is ` an invaluable resource for specialists in lychnology, art history, and archaeology. Front cover: Detail of cat. 86 BUSSIÈRE AND LINDROS WOHL Back cover: Cat. 155 Jean Bussière was an associate researcher with UPR 217 CNRS, Antiquités africaines and was also from getty publications associated with UMR 140-390 CNRS Lattes, Ancient Terracottas from South Italy and Sicily University of Montpellier. His publications include in the J. Paul Getty Museum Lampes antiques d'Algérie and Lampes antiques de Maria Lucia Ferruzza Roman Mosaics in the J. Paul Getty Museum Méditerranée: La collection Rivel, in collaboration Alexis Belis with Jean-Claude Rivel. Birgitta Lindros Wohl is professor emeritus of Art History and Classics at California State University, Northridge. Her excavations include sites in her native Sweden as well as Italy and Greece, the latter at Isthmia, where she is still active. -

Session 8A: Joint AIA/SCS Colloquium (Inter-) Regional Networks in Hellenistic Eurasia
278 ARCHAEOLOGICAL INSTITUTE OF AMERICA Session 8A: Joint AIA/SCS Colloquium (Inter-) Regional Networks in Hellenistic Eurasia Organizer: Talia Prussin, University of California, Berkeley, and Jeremy Simmons, Columbia University Colloquium Overview Statement The Hellenistic world, the long neglected in-between flanked by Classical Greece and Rome, is beginning to see a renaissance of scholarly interest. Helle- nistic history has finally moved away from the study of whole kingdoms through top-down Hellenization toward nuanced regional histories and histories of inter- regional connectivity. New tools in the ancient historian’s toolkit, such as digital mapping and network analysis, can elucidate connections previously invisible in the traditional narrative of the Hellenistic period. This panel has two principal aims: (1) to explore the mechanisms that pro- moted connectivity both regionally and across vast distances within this broader Eurasian “Hellenistic” and (2) to address the burgeoning field of research on the less-studied portions of the Hellenistic world. Accordingly, the papers presented in this panel will look at the role of various types of networks, whether commer- cial, institutional, or spatial, in this broader “Hellenistic.” Individual papers go beyond the traditional chronologies and geographies of the Hellenistic period, evaluating regional case studies from Asia Minor, Mesopotamia, and northwest- ern India between the fourth century B.C.E. and second century C.E. Each paper addresses these Hellenistic phenomena from different perspectives, from trade networks within a single Hellenistic polis to commercial links spanning thousands of kilometers. Panelist #1 (“Transitional Spaces and Connective Tissues: Harbor Dynamics in Hellenistic Asia Minor”) examines the regional connections between Hellenistic poleis in Asia Minor and their associated ports. -
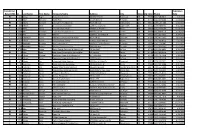
Installers.Pdf
Initial (I) or Expiration Recert (RI) Id Last Name First Sept Name Company Name Address City State Zip Code Phone Date RI 212 Abbas Roger Abbas Construction 6827 NE 33rd St Redmond OR 97756 (541) 548-6812 2/3/2024 I 2888 Adair Benjamin Septic Pros 1751 N Main st Prineville OR 97754 (541) 359-8466 5/10/2024 RI 727 Adams Kenneth Ken Adams Plumbing Inc 906 N 9th Ave Walla Walla WA 99362 (509) 529-7389 6/23/2023 RI 884 Adcock Dean Tri County Construction 22987 SE Dowty Rd Eagle Creek OR 97022 (503) 948-0491 9/25/2023 I 2592 Adelt Georg Cascade Valley Septic 17360 Star Thistle Ln Bend OR 97703 (541) 337-4145 11/18/2022 I 2994 Agee Jetamiah Leavn Trax Excavation LLC 50020 Collar Dr. La Pine OR 97739 (541) 640-3115 9/13/2024 I 2502 Aho Brennon BRX Inc 33887 SE Columbus St Albany OR 97322 (541) 953-7375 6/24/2022 I 2392 Albertson Adam Albertson Construction & Repair 907 N 6th St Lakeview OR 97630 (541) 417-1025 12/3/2021 RI 831 Albion Justin Poe's Backhoe Service 6590 SE Seven Mile Ln Albany OR 97322 (541) 401-8752 7/25/2022 I 2732 Alexander Brandon Blux Excavation LLC 10150 SE Golden Eagle Dr Prineville OR 97754 (541) 233-9682 9/21/2023 RI 144 Alexander Robert Red Hat Construction, Inc. 560 SW B Ave Corvallis OR 97333 (541) 753-2902 2/17/2024 I 2733 Allen Justan South County Concrete & Asphalt, LLC PO Box 2487 La Pine OR 97739 (541) 536-4624 9/21/2023 RI 74 Alley Dale Back Street Construction Corporation PO Box 350 Culver OR 97734 (541) 480-0516 2/17/2024 I 2449 Allison Michael Deschutes Property Solultions, LLC 15751 Park Dr La Pine OR 97739 (541) 241-4298 5/20/2022 I 2448 Allison Tisha Deschutes Property Solultions, LLC 15751 Park Dr La Pine OR 97739 (541) 241-4298 5/20/2022 RI 679 Alman Jay Integrated Water Services 4001 North Valley Dr.