Improved Ionic Model of Liquid Uranium Dioxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

260-280 Osipian.Indd
Le Bourgeois Gentilhomme Political Corruption of Russian Doctorates Ararat L. Osipian Abstract: This paper addresses the issue of doctorates for sale in the Russian Federation. It focuses specifically on the practice of conferring fake or unearned doctoral degrees to elected politicians and other public officials. It assembles and analyzes a database of doctoral degrees, academic ranks, memberships in the academies, awards, decorations, and titles held by the members of the Russian government, the Federation Council and the State Duma. In theory, doctorates are needed to pursue scholarship and research. In practice, however, doctorates offer to their holders some indirect or intangible benefits, among which are public recognition and respect. These can then be transformed into direct and tangible benefits through different means, including the electoral process. Accordingly, it may be the case that Russian political elites abuse the existing system of conferring doctorates in order to improve their image, and use the degrees as status-symbols in the highly ceremonial society of modern Russia. A Le Bourgeois Gentilhomme (The Bourgeois Gentleman) is a comédie-ballet in five acts by Molière, first presented on October 14, 1670. Keywords: corruption, dissertations, doctorates, Duma, elites, politicians, Russia oliticians and other popular figures traditionally deploy verbal distinctions, high vis- Pibility, and publicity in an attempt to attract the public’s attention and the votes of the electorate. The reputations of these public figures are often based on their entourage, decorations, titles, and high academic degrees. Historically, societal elites sought distinc- tions through music, poetry, clothing, bravery in battle, and so on. In China, yellow dye Ararat L. -

Azerbaijani MP: Cinematographer Ibrahimbeyov Is World-Famous Figure in Art and Must Not Lose Objectivity Authors: Aliyev, M
EBSCOhost http://web.ebscohost.com.offcampus.lib.washington.edu/ehost/delivery?... Search History Search Search Terms Search Options Last Run Via Results ID# S2 TX Ibrahimbeyov OR TX Limiters - Publication Date: Interface -15 Ibragimbekov 20000101-20131231 EBSCOhost Search modes - Boolean/Phrase Research Databases Search Screen - Advanced Search Database - Newspaper Source Plus S1 TX Ibrahimbeyov OR TX Search modes - Boolean/Phrase Interface -15 Ibragimbekov EBSCOhost Research Databases Search Screen - Advanced Search Database - Newspaper Source Plus Record: 1 Title: Azerbaijani MP: Cinematographer Ibrahimbeyov is world-famous figure in art and must not lose objectivity Authors: Aliyev, M. Source: Trend News Agency, 04/06/2013 Document Type: Article Accession Number: 2W63243147410 Database: Newspaper Source Plus Azerbaijani MP: Cinematographer Ibrahimbeyov is world-famous figure in art and must not lose objectivity ~~~~~~~~ M. Aliyev April 06--Rustam Ibrahimbeyov is a world-famous figure in art and he must not lose his objectivity, Ana Vatan party chairman, MP Fazail Agamali told Trend today. Agamali was commenting on Ibrahimbeyov's interview with Rosbalt news agency. "He was not with his people during the most difficult days of Azerbaijan's history," Agamali said. "We did not see Ibrahimbeyov in the most active period of the liberation movement. We did not see him among the protesters during the bloody events of January 20." 1 of 59 8/5/2013 1:47 PM EBSCOhost http://web.ebscohost.com.offcampus.lib.washington.edu/ehost/delivery?... The mentioning of parallels between Rustam Ibrahimbeyov and Akram Aylisli by Agamali did not rule out that both of them are governed from a single center. -

Moscow Institute of Physics and Technology (National Research University)
Moscow Institute of Physics and Technology (National Research University) Year of foundation: 1951 Total students: 7 561 / Foreign students: 1 088 Faculties: 12 / Departments: 130 Teachers: 2 069 Professors Associate Professors Doctors of Science Candidates of Science Foreign teachers 276 271 598 879 52 Main educational programmes for foreigners: 61 Bachelor's programme Master's programme Training of highest qualification Specialist programme 28 33 personnel Additional educational programs for foreigners: 4 Russian as a foreign language Other programmes Pre-university training programmes Short programmes 2 2 Moscow Institute of Physics and Technology (National Research University) (MIPT) known informally as Phystech, is a leading Russian university which trains specialists in theoretical and applied physics, applied mathematics and related disciplines. Most of its buildings are in Dolgoprudny (5 km away from Moscow). Some buildings are located in Zhukovsky (40 km away from Moscow) and in the capital itself. MIPT’s so-called “Phystech System” is a unique tradition, an educational legacy, aimed at preparing highly qualified specialists, who are worldwide demanded in key fields of science. Pyotr Kapitsa, Nobel laureate in physics and one of the founding fathers of MIPT, in 1946 outlined the following basic principles of the Phystech System: Leading scientists from key institutions (such as universities, research centers and commercial knowledge-based organization where students do research and write their theses) shall be involved in student education using the high-tech equipment of these institutions. Training in key institutions implies an individual approach to each student. Each second-third year student shall be involved in scientific work. Upon graduation, students shall be able to apply contemporary methods of theoretical and experimental research and possess ample engineering knowledge to efficiently meet relevant technical challenges. -

9783319006963.Pdf
Springer Proceedings in Physics Volume 147 For further volumes: www.springer.com/series/361 Stéphane Sebban r Julien Gautier r David Ros r Philippe Zeitoun Editors X-Ray Lasers 2012 Proceedings of the 13th International Conference on X-Ray Lasers, 11–15 June 2012, Paris, France Editors Stéphane Sebban David Ros Laboratoire d’Optique Appliquée LPGP ENSTA Université Paris-Sud Palaiseau, France Orsay, France Julien Gautier Philippe Zeitoun Laboratoire d’Optique Appliquée Laboratoire d’Optique Appliquée ENSTA ENSTA Palaiseau, France Palaiseau, France ISSN 0930-8989 ISSN 1867-4941 (electronic) Springer Proceedings in Physics ISBN 978-3-319-00695-6 ISBN 978-3-319-00696-3 (eBook) DOI 10.1007/978-3-319-00696-3 Springer Cham Heidelberg New York Dordrecht London Library of Congress Control Number: 2013948223 © Springer International Publishing Switzerland 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. -

An Incessant Battle for Education
Historia i Polityka No. 24 (31)/2018, pp. 33–46 www.hip.umk.pl ISSN 1899-5160, e-ISSN 2391-7652 DOI: http://dx.doi.org/10.12775/HiP.2018.009 Dagmara MOSK WA Polish Academy of Sciences, Institute of Political Studies, Warszawa, Poland Russia: An Incessant Battle for Education Rosja: nieustająca walka o edukację • A bst ra kt • • A bst ract • Tematem niniejszego artykułu są zmiany The presented paper discusses the changes in w rosyjskiej oświacie, które miały miejsce w la- the Russian education system that took place tach 2013–2016, a zatem w okresie prezyden- in the years 2013–2016, during the presidency tury Władimira Putina. Przedmiotem badań of Vladimir Putin. The analysis encompasses są zarówno przeobrażenia w systemie edukacji all dimensions of changes in the education sys- na poziomie kadrowym (zastąpienie Dmitrija tem: personnel changes (replacement of Dmitri Liwanowa przez Olgę Wasilijewą na stanowi- Livanov by Olga Vasil’eva in the position of the sku Ministra Edukacji i Nauki FR), formalnym RF Minister of Education and Science), formal (powstanie nowej: Koncepcji nowego komplek- ones (development of the new: Concept of a new su edukacyjno-metodycznego w zakresie naucza- educational – methodological complex for teach- nia historii ojczystej), instytucjonalnym (refor- ing national history), institutional (reform of the ma Rosyjskiej Akademii Nauk), jak i jakościo- Russian Academy of Sciences) and finally also wym (wprowadzenie nowych podręczników do qualitative changes (introduction of new histo- historii). W artykule podjęto próbę pokazania, ry textbooks). The article attempts to show that że wymienione zmiany stanowią egzemplifika- the above changes are in fact manifestations of cję zaostrzenia kontroli państwa nad oświatą tightening state control over education and its oraz traktowania jej jako narzędzia kreowania treatment as a vehicle to create the historical polityki historycznej Federacji Rosyjskiej (FR). -
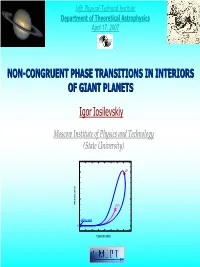
Plasma Phase Transition Concept Hydrogen (S = Const) 3,2 Эксперимент (S = Const) 3,0 Кормер И Др
Ioffe Physical‐Technical Institute Department of Theoretical Astrophysics April 17, 2007 NON-CONGRUENT PHASE TRANSITIONS IN INTERIORS OF GIANT PLANETS Igor Iosilevskiy Moscow Institute of Physics and Technology (State University) CP SURE ES R P PCP Melting point 0 TEMPERATURE 1967 Nature 215 (1967); Internal Structure and Energy Emission of Jupiter R. Smoluchowski Princeton University Princeton, New Jersey Jupiter emits much more energy than it absorbs. Explanations of the source of this heat depend upon our knowledge of its interior and of the behavior of condensed matter at very high temperatures and pressures. 1968 -1970 Norman & Starostin, Plasma Phase Transition Concept Hydrogen (S = const) 3,2 Эксперимент (S = const) 3,0 Кормер и др. 1972 1972 2,8 2,6 3 Mbar } a 2,4 Kormer et al. (Russian Nuclear Center (Sarov)), /GP 2,2 Density jump in quasi‐isentropic compression of hydrogen lg {P 2,0 (P~ 3 Mbar) 1,8 ? ‐ Plasma Phase Transition ‐ ? 1,6 1,4 -0,1 0,0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 lg {ρ/(g/cc)} 1977 Voyager spacecrafts mission to Saturn ‐ Launch (1977) ‐ Start of Jupiter exploring (1979) ‐ Start of Saturn exploring (1980) ‐ Voyager mission is still valid (2007) Phase Separation in Giant Planets: Jonathan J. FORTNEY, William B. HUBBARD Icarus, 164 (1) 2003 Atmospheric elemental abundances in Jupiter and Saturn (mass fractions) Element SOLAR JUPITER SATURN SATURN Galileo Voyager revised H 0.736 0.742 0.92 0.76 He 0.249 0.231 ± 0.04 0.06 ± 0.05 0.215 ± 0.035 1977 Stevenson & Salpeter, Astrophysical Journal, Suppl. -

Russian Academy 2006.Pdf (5.402Mb)
The Russian Academy of Sciences, 2006 Update With an historical introduction by the President of the Academy Iuri S. Osipov From Yu.S. Osipov's book «Academy of Sciences in the History of the Russian State» Moscow, «NAUKA», 1999 The creation of the Academy of Sciences is directly connected with Peter the Great’s reformer activities aimed at strengthening the state, its economic and political independence. Peter the Great understood the importance of scientific thought, education and culture for the prosperity of the country. And he started acting “from above”. Under his project, the Academy was substantially different from all related foreign organizations. It was a state institution; while on a payroll, its members had to provide for the scientific and technical services of thee state. The Academy combined the functions of scientific research and training, having its own university and a high school. On December 27, 1725, the Academy celebrated its creation with a large public meeting. This was a solemn act of appearance of a new attribute of Russian state life. Academic Conference has become a body of collective discussion and estimation of research results. The scientists were not tied up by any dominating dogma, were free in their scientific research, and took an active part in the scientific opposition between the Cartesians and Newtonians. Possibilities to publish scientific works were practically unlimited. Physician Lavrentii Blumentrost was appointed first President of the Academy. Taking care of bringing the Academy’s activities to the world level, Peter the Great invited leading foreign scientists. Among the first were mathematicians Nikolas and Daniil Bornoulli, Christian Goldbach, physicist Georg Bulfinger, astronomer and geographer J.Delille, historian G.F.Miller. -

Russia's Science-Election Chaos
IN FOCUS NEWS Experimental Reactor (ITER) in southern exodus, says Chapman, but some top-level staff so more people are going to consider countries France, for which JET is a test bed. Both tasks members have accepted positions elsewhere, in mainland Europe — particularly Germany, got harder in January, when the UK govern- and candidates have rejected job offers, citing where the funding is so good.” ment announced that, as part of the country’s questions over JET’s future. Germany’s research and development withdrawal from the EU, it would also pull out Despite these uncertainties, Chapman thinks spending relative to its gross domestic product of the European Atomic Energy Community that the government understands what is at is among the highest in Europe. (Euratom), the body that disseminates EU stake and says that Zerial expects to see an increase in appli- fusion funding and manages the United King- “There’s a it has been respon- cations to the large, international graduate dom’s membership of ITER. time window sive. But the United school that is jointly run by his institute with The decision wasn’t a complete surprise, says beyond which Kingdom’s fusion the Technical University of Dresden, as well as Chapman. But it came without warning or an community needs in applications for postdoc and group-leader obvious plan for how to maintain the United the disquiet will a concrete signal positions. “It’ll be to our benefit.” Kingdom’s fusion programme after the nation ratchet up.” from the govern- But Brexit will hurt European science in the leaves Euratom. -
Dear Colleagues, We Are Pleased to Inform You That the Joint
Dear colleagues, We are pleased to inform you that the Joint Institute for High Temperatures of the Russian Academy of Sciences (JIHT RAS), Moscow, and the GSI Helmholtzzentrum für Schwerionenforschung (GSI), Darmstadt, are organizing the Fifth Workshop on Plasma Physics with Intense Laser and Heavy Ion Beams. This workshop is a continuation of serial meetings within the framework of the Memorandum of Understanding between GSI, EMMI and JIHT RAS for cooperation in the investigation of hot and dense plasmas and in the development for accelerator and laser based plasma physics in GSI and JIHT RAS, signed in November 2008. The workshop will take place on October 8–9, 2012, at JIHT RAS, Room 230, Izhorskaya 13 Bldg 2, Moscow 125412, Russia. The workshop is focused on the research field of laser and heavy-ion beams interaction with matter, in particular the creation and exploration of matter at extreme conditions, including inertial fusion and astrophysical implications. The research activities in these fields have long term collaborations between GSI, Germany, and leading Russian Institutions: JIHT RAS, Institute of Theoretical and Experimental Physics (ITEP), Moscow, Institute of Problems of Chemical Physics (IPCP) RAS, Chernogolovka, Prokhorov General Physics Institute (GPI) RAS, Moscow, Lebedev Physical Institute (LPI) RAS, Moscow, VNIITF, Snezhinsk, and VNIIEF, Sarov. One of the objects of the workshop is to survey the current progress in the Russian–German collaboration of the involved Institutions in the research on Plasma Physics with Intense Laser and Heavy Ion Beams. The main topics of the workshop include: 1. Status of the scientific program with femtosecond and nanosecond – PHELIX laser pulses in GSI. -
Language, Historiography and Economy in Late- and Post-Soviet Leningrad
Language, Historiography and Economy in late- and post-Soviet Leningrad: “the Entire Soviet People Became the Authentic Creator of the Fundamental Law of their Government.” Xenia A. Cherkaev Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2015 © 2015 Xenia A. Cherkaev All Rights Reserved Abstract: Language, Historiography and Economy in late- and post-Soviet Leningrad: “the Entire Soviet People Became the Authentic Creator of the Fundamental Law of their Government.” Xenia A. Cherkaev This dissertation is about holes. It begins by analyzing the proverbial “hole in the fence” at late-Soviet enterprises: the way that workers pragmatically employed the planned economy's distribution rules by actions that were both morally commendable and questionably legal. It then analyzes the omission of this hole in perestroika economic analysis, which devoted surprisingly little attention to enterprises' central role in providing welfare and exerting social control, or to employees' pragmatic employment of the enterprises' rules. This analytic hole is compounded by a historiographic one: by the omission of the post-1956 omission of Stalin's name from public mention. Framing the perestroika reforms against “Stalinism,” perestroika-era texts typically trace the start of de-Stalinization to Khrushchev's “Cult of Personality” speech, after which Stalin's name disappeared from textbooks; rather than to the post-1953 reforms that fundamentally restructured labor, economic and punitive institutions to create characteristically late-Soviet methods of retaining and motivating labor: including the widespread disciplinary lenience that allowed workers to pragmatically employ enterprise rules. -

Michael Graetzel Spoke About Sim- Conference
15 years – energy of the future, created by knowledge ANNUAL REPORT ANNUAL REPORT Contents 1. 2. 3. 4. The Association The Global Energy Prize The Association’s programs Sustainable development Annexes Highlights 4 History of the Association 28 About the Prize 40 About the programs 60 Governance system 70 Annex 1. About the report 104 Key events 12 Members of the Association 30 The Global Energy Prize The Energy of Start Internal control and audit 79 International Award Committee 41 Program 61 Annex 2. Organizational structure 31 Implementation of IT solutions 80 Global coverage 20 GRI content index 105 Nomination process 43 The Energy of Youth Mission and values 33 Contest 62 HR policy 82 Greetings from the president Annex 3. Nomination cycle 44 Financial statements 2017 108 of the Association 24 Strategic development priorities 34 The Energy of Breakthrough Changes related work 83 Nominating persons 46 Contest 63 Annex 4. Financial results 2017 36 Relations with stakeholders 84 Glossary 114 Independent international The Energy of Thought Work with the Prize laureates experts pool 46 Symposium 64 Annex 5. and programs’ winners 85 Contact information 115 National Prize support The Energy of Knowledge committees 47 Program 64 Annex 6. Feedback form 116 Nomination cycle results 2017 48 The Energy of Education Program 65 The Prize laureates 50 The Energy of Inspiration The Prize development 56 Program 66 Position in the sector 57 The Energy of Words Contest 67 Highlights ANNUAL REPORT Key performance indicators for 15 years Geography of nominees -

Kremlin Kontrol
Cover The cover focuses on the three faces most responsible for the institution of control measures over civilian and military affairs in Russia: President Vladimir Putin, Defense Minister Sergey Shoygu, and Chief of the General Staff Valery Gerasimov. The title of Kremlin Kontrol is used to stress two things, that it is from leadership directives that everything of significance in regard to Russian national interests is decided or acted upon; and that the system of control over civilian and military issues is designed to help neutralize feelings of insecurity or vulnerability among Kremlin elites. Cover image attribution: Vladimir Putin: Kremlin.ru [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0) or CC BY 4.0 (http://creativecommons.org/licenses/by/4.0)], via Wikimedia Commons General Gerasimov: By Министерство Обороны Российской Федерации [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons Sergey Shoygu: By Vitaly V. Kuzmin (http://vitalykuzmin.net/?q=node/512) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0) or CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons The Foreign Military Studies Office (FMSO) is a component of the US Army's Training and Doctrine Command (TRADOC) G2 that does guidance-driven, unclassified research and analysis of the foreign perspective of the operational environment. The views expressed in this document are those of the author and do not reflect the official policy or position of the Department of Defense or the US government. TABLE OF CONTENTS TABLE OF CONTENTS ......................................................................