Management of Benign Central Airway Obstruction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Current Management and Outcome of Tracheobronchial Malacia and Stenosis Presenting to the Paediatric Intensive Care Unit
Intensive Care Med 52001) 27: 722±729 DOI 10.1007/s001340000822 NEONATAL AND PEDIATRIC INTENSIVE CARE David P.Inwald Current management and outcome Derek Roebuck Martin J.Elliott of tracheobronchial malacia and stenosis Quen Mok presenting to the paediatric intensive care unit Abstract Objective: To identify fac- but was not related to any other fac- Received: 10 July 2000 Final Revision received: 14 Oktober 2000 tors associated with mortality and tor. Patients with stenosis required a Accepted: 24 October 2000 prolonged ventilatory requirements significantly longer period of venti- Published online: 16 February 2001 in patients admitted to our paediat- latory support 5median length of Springer-Verlag 2001 ric intensive care unit 5PICU) with ventilation 59 days) than patients tracheobronchial malacia and with malacia 539 days). stenosis diagnosed by dynamic con- Conclusions: Length of ventilation Dr Inwald was supported by the Medical Research Council. This work was jointly trast bronchograms. and bronchographic diagnosis did undertaken in Great Ormond Street Hos- Design: Retrospective review. not predict survival. The only factor pital for Children NHSTrust, which re- Setting: Tertiary paediatric intensive found to contribute significantly to ceived a proportion of its funding from the care unit. mortality was the presence of com- NHSExecutive; the views expressed in this Patients: Forty-eight cases admitted plex cardiac and/or syndromic pa- publication are those of the authors and not to our PICU over a 5-year period in thology. However, patients with necessarily those of the NHSexecutive. whom a diagnosis of tracheobron- stenosis required longer ventilatory chial malacia or stenosis was made support than patients with malacia. -

Posterior Glottic Stenosis in Adults
Original Articles Posterior Glottic Stenosis in Adults Michael Wolf MD, Adi Primov-Fever MD, Yoav P. Talmi MD FACS and Jona Kronenberg MD Department of Otorhinolaryngology & Head Neck Surgery, Sheba Medical Center, Tel Hashomer, Israel Affiliated to Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel Key words: larynx, vocal cords, stenosis, fixation, laryngoplasty Abstract entiation between vocal cord paralysis and fixation [3,4], although Background: Posterior glottic stenosis is a complication of some patients survived complex central nervous system and prolonged intubation, manifesting as airway stenosis that may mimic peripheral organ injuries, long courses of mechanical ventilation, bilateral vocal cord paralysis. It presents a variety of features that oral or nasal intubation, and nasogastric tubing. These may result mandate specific surgical interventions. in combined pathologies of both paralysis and fixation, or they Objectives: To summarize our experience with PSG and its working diagnosis. may mask the alleged simplicity of making a diagnosis based Methods: We conducted a retrospective review of a cohort of solely on clinical grounds. The extent of scarring dictates the adult patients with PGS operated at the Sheba Medical Center extent of surgical intervention. While endoscopic lysis of the scar between 1994 and 2006. is appropriate for grade 1, complex laryngoplasty procedures are Results: Ten patients were diagnosed with PGS, 6 of whom mandated for grades 2 to 4 [3]. also had stenosis at other sites of -

Pulmonary Manifestations of Collagen Vascular Diseases
July 2009; Volume 3(1) Review Article Pulmonary Manifestations of Collagen Vascular Diseases C. P. Dokwal1 The collagen vascular diseases (CVDs) include a patients with long-standing SLE in the past 5, however recent heterogeneous group of chronic inflammatory HRCT series reveal that about 1/3rd of patients with SLE immunologically-mediated systemic diseases, such as have ILD, most having early sub clinical disease.6 It usually rheumatoid arthritis (RA), systemic lupus erythematosus develops insidiously and is associated with recurrent pleural (SLE), systemic sclerosis (SSc), Sjogren's syndrome (SS), effusions. polymyositis (PM)/dermatomyositis (DM), and mixed Chest radiographs typically show diffuse alveolar opacities. connective tissue disease (MCTD). They present with a wide HRCT of chest often reveals a cellular, fibrotic, or mixed non- range of clinical manifestations. The clinical features in specific interstitial pneumonia (NSIP) pattern.7 Usual CVDs frequently overlap causing much clinical confusion. interstitial pneumonia (UIP) and lymphoid pneumonia (LIP), The lung is frequently affected in CVDs and is the cause of particularly in those with associated secondary Sjogren's significant morbidity and mortality. The common pulmonary syndrome, have also been described.7, 8 Rarely, organizing manifestations include pleural disease, pulmonary fibrosis, pneumonia has also been reported. bronchiolitis obliterans, obliterans, organizing pneumonia, Diffuse Alveolar Haemorrhage bronchiectasis, aspiration pneumonia, and diaphragmatic weakness. -

ERS Statement on Tracheomalacia and Bronchomalacia in Children
ERS OFFICIAL DOCUMENT ERS STATEMENT ERS statement on tracheomalacia and bronchomalacia in children Colin Wallis1,EfthymiaAlexopoulou2,JuanL.Antón-Pacheco3,JayeshM.Bhatt 4, Andrew Bush5,AnneB.Chang6,7,8,Anne-MarieCharatsi9, Courtney Coleman10, Julie Depiazzi11, Konstantinos Douros12,ErnstEber13,MarkEverard14, Ahmed Kantar15,IanB.Masters6,7,FabioMidulla16, Raffaella Nenna 16,17, Derek Roebuck18, Deborah Snijders19 and Kostas Priftis12 @ERSpublications This statement provides a comprehensive review of the causes, presentation, recognition and management of children with tracheobronchomalacia written by a multidisciplinary Task Force in keeping with ERS methodology http://bit.ly/2LPTQCk Cite this article as: Wallis C, Alexopoulou E, Antón-Pacheco JL, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J 2019; 54: 1900382 [https://doi.org/10.1183/13993003.00382- 2019]. ABSTRACT Tracheomalacia and tracheobronchomalacia may be primary abnormalities of the large airways or associated with a wide variety of congenital and acquired conditions. The evidence on diagnosis, classification and management is scant. There is no universally accepted classification of severity. Clinical presentation includes early-onset stridor or fixed wheeze, recurrent infections, brassy cough and even near-death attacks, depending on the site and severity of the lesion. Diagnosis is usually made by flexible bronchoscopy in a free-breathing child but may also be shown by other dynamic imaging techniques such as low-contrast volume bronchography, computed tomography or magnetic resonance imaging. Lung function testing can provide supportive evidence but is not diagnostic. Management may be medical or surgical, depending on the nature and severity of the lesions, but the evidence base for any therapy is limited. While medical options that include bronchodilators, anti-muscarinic agents, mucolytics and antibiotics (as well as treatment of comorbidities and associated conditions) are used, there is currently little evidence for benefit. -

Tracheobronchomalacia and Excessive Dynamic Airway Collapse
Blackwell Publishing AsiaMelbourne, AustraliaRESRespirology1323-77992006 Blackwell Publishing Asia Pty Ltd? 2006114388406Review ArticleTBM and EDACSD Murgu and HG Colt Respirology (2006) 11, 388–406 doi: 10.1111/j.1400-1843.2006.00862.x REVIEW ARTICLE Tracheobronchomalacia and excessive dynamic airway collapse Septimiu D. MURGU AND Henri G. COLT Pulmonary and Critical Care Medicine, Department of Medicine, University of California School of Medicine, Irvine, CA, USA Tracheobronchomalacia and excessive dynamic airway collapse MURGU SD, COLT HG. Respirology 2006; 11: 388–406 Abstract: Tracheobronchomalacia and excessive dynamic airway collapse are two separate forms of dynamic central airway obstruction that may or may not coexist. These entities are increasingly recognized as asthma and COPD imitators. The understanding of these disease processes, however, has been compromised over the years because of uncertainties regarding their definitions, patho- genesis and aetiology. To date, there is no standardized classification, diagnosis or management algorithm. In this article we comprehensively review the aetiology, morphopathology, physiology, diagnosis and treatment of these entities. Key words: airflow dynamics, bronchomalacia, excessive dynamic airway collapse, tracheobron- chomalacia, tracheomalacia. INTRODUCTION first is that most published studies are case series and retrospective descriptions, many of which report a The purpose of this systematic review is to clarify con- single investigator’s experience with diagnosis and founding issues pertaining to the definition, patho- management. In fact, it is puzzling that despite the physiology, histopathology, aetiology, diagnosis, relative frequency with which TBM and EDAC are pre- classification and treatment of acquired and idio- sumably encountered, multi-institutional or prospec- pathic forms of adult tracheobronchomalacia (TBM) tive studies have not been published. -

Series of Laryngomalacia, Tracheomalacia, and Bronchomalacia Disorders and Their Associations with Other Conditions in Children
Pediatric Pulmonology 34:189-195 (2002) Series of Laryngomalacia, Tracheomalacia, and Bronchomalacia Disorders and Their Associations With Other Conditions in Children I.B. Masters, MBBS, FRACP,1* A.B. Chang, PhD, FRACP,2 L. Patterson, MBBS, FANZCAC,1 С Wainwright, MD, FRACP,1 H. Buntain, MBBS,1 B.W. Dean, MSC,1 and P.W. Francis, MD, FRACP1 Summary. Laryngomalacia, bronchomalacia, and tracheomalacia are commonly seen in pediatric respiratory medicine, yet their patterns and associations with other conditions are not well-understood. We prospectively video-recorded bronchoscopic data and clinical information from referred patients over a 10-year period and defined aspects of interrelationships and associations. Two hundred and ninety-nine cases of malacia disorders (34%) were observed in 885 bronchoscopic procedures. Cough, wheeze, stridor, and radiological changes were the most common symptoms and signs. The lesions were most often found in males (2:1) and on the left side (1.6:1). Concomitant malacia lesions ranged from 24%forlaryngotracheobronchomalaciato 47% for tracheobronchomalacia. The lesions were found in association with other disorders such as congenital heart disorders (13.7%), tracheo-esophageal fistula (9.6%), and various syndromes (8%). Even though the understanding of these disorders is in its infancy, pediatricians should maintain a level of awareness for malacia lesions and consider the possibility of multiple lesions being present, even when one symptom predominates or occurs alone. Pediatr Pulmonol Pediatr Pulmonol. 2002; 34:189-195. © 2002 wiiey-Liss. inc. Key words: laryngomalacia; tracheomalacia; bronchomalacia; malacia disorders; syndromes. INTRODUCTION The aim of this report is to describe an extensive experience of various forms of laryngomalacia, tracheo Tracheomalacia, bronchomalacia, and laryngomalacia malacia, and bronchomalacia and explore some of the disorders are commonly seen in tertiary pediatric respira interrelationships that exist between these conditions with tory practice. -

Idiopathic Subglottic Stenosis: a Review
1111 Editorial Section of Interventional Pulmonology Idiopathic subglottic stenosis: a review Carlos Aravena1,2, Francisco A. Almeida1, Sanjay Mukhopadhyay3, Subha Ghosh4, Robert R. Lorenz5, Sudish C. Murthy6, Atul C. Mehta1 1Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA; 2Department of Respiratory Diseases, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile; 3Department of Pathology, Robert J. Tomsich Pathology and Laboratory Medicine Institute, 4Department of Diagnostic Radiology, 5Head and Neck Institute, 6Department of Thoracic and Cardiovascular Surgery, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA Contributions: (I) Conception and design: C Aravena, AC Mehta; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: C Aravena, AC Mehta; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Atul C. Mehta, MD, FCCP. Lerner College of Medicine, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Ave, A-90, Cleveland, OH 44195, USA. Email: [email protected]. Abstract: Idiopathic subglottic stenosis (iSGS) is a fibrotic disease of unclear etiology that produces obstruction of the central airway in the anatomic region under the glottis. The diagnosis of this entity is difficult, usually delayed and confounded with other common respiratory diseases. No apparent etiology is identified even after a comprehensive workup that includes a complete history, physical examination, pulmonary function testing, auto-antibodies, imaging studies, and endoscopic procedures. This approach, however, helps to exclude other conditions such as granulomatosis with polyangiitis (GPA). It is also helpful to characterize the lesion and outline management strategies. -
Paediatric Airway Problems
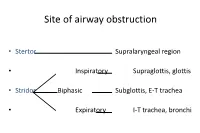
Site of airway obstruction • Stertor Supralaryngeal region • Inspiratory Supraglottis, glottis • Stridor Biphasic Subglottis, E-T trachea • Expiratory I-T trachea, bronchi Investigation of Stridor • History • Examination • Radiological investigations • Endoscopy History • Neonatal intubation • Stridor / stertor • Voice / cry • Cough / cyanotic attacks • Feeding difficulties • Failure to thrive Examination • Pallor, cyanosis • Tracheal tug, sternal recession, intercostal / subcostal recession • Pectus excavatum, Harrison’s sulci • Stridor / stertor • Voice / cry • Cough Site of Airway Obstruction Stridor Voice Cough Supralaryngeal Stertor Muffled - region Supraglottis, Inspiratory Hoarse Barking glottis Subglottis, E-T Biphasic Normal Brassy trachea I-T trachea, Expiratory Normal + bronchi Site of Airway Obstruction • Pharyngeal (stertor): - worse when asleep • Laryngeal, tracheal or bronchial (stridor): - worse when awake, especially if stressed Radiological investigations • Soft-tissue lateral neck X-ray • PA chest X-ray • Cincinnati (high-kv filter) view • ? Barium swallow • ? Bronchogram Endoscopy • The definitive investigation for a child with stridor Endoscopy • Awake flexible fibreoptic laryngoscopy • Suitable for infants using simple restraint • Screening investigation for laryngomalacia • May be helpful in assessing vocal cord palsy • But only gives a view of the supraglottis • Does not exclude coexisting lower airway pathology Endoscopy • Microlaryngoscopy + Bronchoscopy • “The Gold Standard” Total airway endoscopies at GOS 9,341 -

Imaging of the Trachea
Keynote Lecture Series Imaging of the trachea Jo-Anne O. Shepard, Efren J. Flores, Gerald F. Abbott Division of Thoracic Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA Correspondence to: Jo-Anne O. Shepard, MD. Thoracic Imaging, Department of Radiology, Massachusetts General Hospital, Founders 202, 55 Fruit Street, Boston, MA 02114, USA. Email: [email protected]. Numerous benign and malignant tracheal diseases may affect the trachea primarily and secondarily. While the posterior anterior (PA) and lateral chest radiograph is the conventional study for initial evaluation of the trachea and central airways, findings may not always be apparent on conventional radiographs, and further evaluation with cross sectional imaging is usually necessary. Computed tomography (CT) is the imaging modality of choice for imaging the trachea and bronchi. Familiarity with the imaging appearances of the normal and diseased trachea will enhance diagnostic evaluation. Keywords: Trachea; tracheal stenosis; tracheal tumor; tracheomalacia Submitted Jan 09, 2018. Accepted for publication Feb 27, 2018. doi: 10.21037/acs.2018.03.09 View this article at: http://dx.doi.org/10.21037/acs.2018.03.09 Introduction mediastinal structures away from the trachea. Visualization of tracheal abnormalities can also be improved by high The trachea extends from the cricoid cartilage to the kilovoltage technique (140 kV). Evaluation of the trachea carina and usually measures 10–12 cm in length. The extra and central airways should be carefully performed on all thoracic trachea is (2–4 cm in length) and transitions to patients presenting with symptoms of stridor, wheezing, the intrathoracic trachea (6–9 cm in length) as it passes “adult-onset” asthma, hemoptysis or recurrent pneumonia. -

Laryngeal Stenosis and Laryngomalacia Treated with a Silicone Stent in a Prematurely Delivered Child: a Case Report
Journal of Medical Sciences. May 3, 2021 - Volume 9 | Issue 4. Electronic - ISSN: 2345-0592 Medical Sciences 2021 Vol. 9 (4), p. 216-220 Laryngeal stenosis and laryngomalacia treated with a silicone stent in a prematurely delivered child: a case report Jurgita Borodičienė¹, Eglė Žukauskaitė², Matas Kalinauskas², Marius Kašėta³, Andrius Macas¹ ¹ Department of Anesthesiology, Lithuanian University of Health Sciences, Eiveniu str. 2, LT-50009, Kaunas, Lithuania ² Faculty of Medicine, Academy of Medicine, Lithuanian University of Health Sciences, Eiveniu str. 2, LT-50009 Kaunas, Lithuania ³ Department of Otorhinolaryngology, Lithuanian University of Health Sciences, Eiveniu str. 2, LT-50009, Kaunas, Lithuania Abstract Background. Preterm birth is one of the most significant risk factors for infants’ laryngeal stenosis (LS), which can occur as laryngomalacia (LM). LS can be defined as a partial or circumferential narrowing of the endolaryngeal airway and may be congenital or acquired. This condition cause various symptoms such as stridor, dyspnea and respiratory distress, therefore early intubation is required. Most of the patients with LS or LM outgrow their disease, but the rest of them need surgical help. Objectives. The aim of this article is to present a successful surgical treatment for preterm infant with LS. Methods: case presentation and an analysis of literature. Research of articles in “PubMed”, “Google Scholar” databases with keywords used as follows: “Laryngeal stenosis”, “Laryngomalacia”, “Pediatric stent”, “Premature delivery”, “Endolaryngeal microsurgery”. Case presentation. 3-year-old male presented the hospital with diagnosed LM and for laryngeal stent insertion surgery. The patient was born at 26 gestational weeks and was treated for sepsis during the neonatal period, was intubated at birth because of severe respiratory distress, had 6 endolaryngeal microsurgeries (EM) and received Kenalog injections 5 times. -

Supportive Pericardial Suspension for Surgical Airway Management Of
Case report 147 Supportive pericardial suspension for surgical airway management of tracheobronchomalacia in unilateral pulmonary agenesis Tomomi Hasegawaa, Yoshihiro Oshimaa, Yuichi Okatab and Ayako Maruoa Unilateral pulmonary agenesis, a rare developmental Keywords: pericardial suspension, tracheobronchomalacia, unilateral pulmonary agenesis defect of the lung, is often accompanied by a b tracheobronchial stenosis or malacia due to displacement, Departments of Cardiovascular Surgery and Pediatric Surgery, Kobe Children’s Hospital, Kobe, Japan distortion, and compression of the surrounding great vessels. We present two cases of unilateral pulmonary Correspondence to Tomomi Hasegawa, MD, Department of Cardiovascular Surgery, Kobe Children’s Hospital, 1-1-1 Takakuradai, Suma-ku, Kobe, agenesis complicated by tracheobronchial problems that Hyogo 654-0081, Japan were successfully managed surgically with supportive Tel: + 81 78 732 6961; fax: + 81 78 735 0910; e-mail: [email protected] pericardial suspension. Ann Pediatr Surg 11:147–149 c 2015 Annals of Pediatric Surgery. Received 16 October 2014 accepted 5 March 2015 Annals of Pediatric Surgery 2015, 11:147–149 Unilateral pulmonary agenesis (UPA) is a rare develop- Case 1 mental defect of the lung, which has been defined as a A male infant was born with a birth weight of 3362 g at 38 complete defect of the pulmonary parenchyma and artery weeks of gestation. Soon after birth, he was presented with absence of the bronchus. Patients with UPA present with respiratory distress and required immediate intuba- unique anatomic features such as an ipsilateral shift and tion and mechanical ventilation. The infant was success- rotation of the heart and mediastinum to the empty fully extubated after improvement of persistent hemithorax, which results in displacement, distortion, pulmonary hypertension at 25 days of age, but he was and compression of the great vessels and airway. -

Post Tracheostomy and Post Intubation Tracheal Stenosis: Report of 31
BMC Pulmonary Medicine BioMed Central Research article Open Access Post tracheostomy and post intubation tracheal stenosis: Report of 31 cases and review of the literature Nikolaos Zias*1, Alexandra Chroneou1, Maher K Tabba2, Anne V Gonzalez1, Anthony W Gray1, Carla R Lamb1, David R Riker1 and John F Beamis Jr1 Address: 1Department of Pulmonary and Critical Care Medicine, Lahey Clinic Medical Center, Tufts University School of Medicine, Burlington, Massachusetts, USA and 2Department of Pulmonary, Critical Care and Sleep Medicine, Tufts University School of Medicine, Boston, Massachusetts, USA Email: Nikolaos Zias* - [email protected]; Alexandra Chroneou - [email protected]; Maher K Tabba - [email protected]; Anne V Gonzalez - [email protected]; Anthony W Gray - [email protected]; Carla R Lamb - [email protected]; David R Riker - [email protected]; John F Beamis - [email protected] * Corresponding author Published: 21 September 2008 Received: 25 April 2008 Accepted: 21 September 2008 BMC Pulmonary Medicine 2008, 8:18 doi:10.1186/1471-2466-8-18 This article is available from: http://www.biomedcentral.com/1471-2466/8/18 © 2008 Zias et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Severe post tracheostomy (PT) and post intubation (PI) tracheal stenosis is an uncommon clinical entity that often requires interventional bronchoscopy before surgery is considered. We present our experience with severe PI and PT stenosis in regards to patient characteristics, possible risk factors, and therapy.