A Comparative Study on the Effect of Argan Oil Versus Fish Oil on Risk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -

Nutraceutical Potentialities of Tunisian Argan Oil Based on Its
Hanana et al. Lipids in Health and Disease (2018) 17:138 https://doi.org/10.1186/s12944-018-0782-9 RESEARCH Open Access Nutraceutical potentialities of Tunisian Argan oil based on its physicochemical properties and fatty acid content as assessed through Bayesian network analyses Mohsen Hanana1, Hajer Mezghenni2, Rayda Ben Ayed3*, Ali Ben Dhiab4, Slim Jarradi5, Bassem Jamoussi6 and Lamia Hamrouni2 Abstract Background: Argan oil is traditionally produced by cold pressing in South-western Morocco where rural population uses it as edible oil as well as for its therapeutic properties which give them in counterpart valuable income. Given the economical interest of this oil, several attempts of fraudulency have been registered in the world global market leading to loss of authenticity. Our purpose is to launch a program of Tunisian Argan oil valorization since trees from this species have been introduced sixty years ago in Tunisia. The first step was thus to characterize the physicochemical properties and determine the chemical composition of Tunisian Argan oil in order to assess its quality. Methods: Physicochemical parameters of oil quality were determined according to the international standard protocols. Fatty acid content analysis of Argan oils was performed by gas chromatography coupled to mass spectrophotometry. A comparative study was realized among Tunisian, Moroccan and Algerian samples differing also by their extraction procedure. The impact of geographical localisation on the fatty acids composition was studied by statistical and modeling Bayesian analyses. Results: Physicochemical parameters analysis showed interestingly that Tunisian Argan oil could be classified as extra virgin oil. Argan oil is mainly composed by unsaturated fatty acids (80%), mainly oleic and linoleic acid (linoleic acid was positively influenced by the geographical localization (r =0.899,p = 0.038) and the P/S index (r =0.987,p =0.002)) followed by saturated fatty acids (20%) with other beneficial compounds from the unsaponifiable fraction like polyphenols and carotenoids. -

Rejuveniqe Oil Intensive Vs Moroccan Oil
Rejuveniqe Oil Intensive vs Moroccan Oil Blend of rare, pure oils from around the world Silicone polymers, synthetic fragrances, argan oil, food coloring, and preservative 1. Meadow foam Oil 2. Abyssinian Oil 1. Cyclopentasiloxane, 3. Camellia Oleifera Oil 2. Dimethicone 4. Tomato Seed Oil 3. Cyclomethicone 5. Carrot Seed Oil 4. Butylphenyl Methylpropional 6. Lemon Peel Oil 5. Argan Oil 7. Lime Oil 6. Linum Usitatissimum (Linseed ) Extract 8. Bergamot Fruit Oil 7. Parfum (Fragrance) 9. Adansonia Digitata Oil 8. D&C Yellow-11 10. Mauritia Flexuosa Fruit Oil 9. D&C Red-17 11. Coconut Oil 10. Benzyl Benzoate 12. Gardenia Tahitensis Flower Extract 11. Alpha-Isomethyl Ionone 13. Moringa Oleifera Seed Oil 14. Caryocar Brasiliense Fruit Oil 15. Sunflower Seed Unique molecular structure – small molecules Large, synthetic, silicon polymer molecules Small molecules that can penetrate and absorb “The molecules in argan oil are too large to penetrate deep in to hair follicle and skin, making it your hair cuticle. Since it can't penetrate, it actually just weightless. sits on top of your hair. This can be a problem if you're using it when your hair is wet, or if you're using too So compatible that it mimics the body’s own much. Applying the oil to damp strands before drying natural oils. will leave your hair feeling smooth for a while, but over time it will actually dry out your hair. The argan oil Can be added to color, bleach, and perms for winds up creating a barrier on top of your hair, which incredibly healthy, shiny results! blocks out any moisturizer trying to get in.” Mark Townsend, Celebrity hair stylist MONAT sources our oils from around the world, The first 3 ingredients of Moroccan Oil carefully vetting each supplier to ensure that what we (Cyclopentasiloxane, Dimethicone and put into our product line meets the strictest standards Cyclomethicone) are large, synthetic, silicon polymer of potency, purity, sustainability and worker molecules built from repeating chemical units. -

Comparative Study of Argan and Olive Fruits and Oils
435 Comparative study of argan and olive fruits and oils Dalila Demnati1, Sebastián Sánchez2, Rafael Pacheco2, Mohamed Zahar1 & Leopoldo Martínez2 1- Institut Agronomique et Vétérinaire Hassan. BP 6202, 10101, Rabat, Morocco. Email: [email protected] 2- Universidad de Jaén, Campus Las Lagunillas 23071 Jaén, Spain. Email: [email protected] Abstract This study was conducted to compare argan and olive fruits and virgin oils. Dry argan fruits, traditional and semiautomatic extracted argan oils, from roasted and unroasted seeds, from Essaouira’s area, were studied. Morphological characteristics of argan fruit were determined and compared with the ‘Picual’ olive’s ones. The results showed certain similarities between the two fruits. The quality parameters analyzed were acidity and peroxide value, K270, K232 and ΔK, total phenols and oil stability, comparing them with those of ‘Picual’ virgin olive oil. Quality parameters corresponded to the Moroccan Standard for edible virgin argan oil. Traditional argan oil showed the lowest stability whereas semiautomatic edible oil presented the highest one. However, virgin olive oil showed higher phenol content and better oxidative stability than the virgin argan oils. Keywords: Argan, Olive, Fruit, Quality Parameters, Phenols, Oil Stability. Etude comparative des caractéristiques des fruits et des huiles d’argan et d’olive Résumé Cette étude a pour but de comparer le fruit et l’huile d’argan et d’olive vierges. Les fruits secs d’arganier, les huiles d’argan obtenues par un procédé traditionnel ou semi-automatique, à partir d’amandons torréfiés ou non, de la région d’Essaouira, ont été étudiés. Les caractéristiques morphologiques du fruit de l’arganier ont été déterminées et comparées à ceux de l’olive, variété ‘Picual’. -

Fatty Acid Composition of Cosmetic Argan Oil: Provenience and Authenticity Criteria
molecules Article Fatty Acid Composition of Cosmetic Argan Oil: Provenience and Authenticity Criteria Milena BuˇcarMiklavˇciˇc 1, Fouad Taous 2, Vasilij Valenˇciˇc 1, Tibari Elghali 2 , Maja Podgornik 1, Lidija Strojnik 3 and Nives Ogrinc 3,* 1 Science and Research Centre Koper, Institute for Olive Culture, 6000 Koper, Slovenia; [email protected] (M.B.M.); [email protected] (V.V.); [email protected] (M.P.) 2 Centre National De L’énergie, Des Sciences Et Techniques Nucleaires, Rabat 10001, Morocco; [email protected] (F.T.); [email protected] (T.E.) 3 Department of Environmental Sciences, Jožef Stefan Institute, Jamova cesta 39, 1000 Ljubljana, Slovenia; [email protected] * Correspondence: [email protected]; Tel.: +386-1588-5387 Academic Editor: George Kokotos Received: 17 July 2020; Accepted: 3 September 2020; Published: 7 September 2020 Abstract: In this work, fatty-acid profiles, including trans fatty acids, in combination with chemometric tools, were applied as a determinant of purity (i.e., adulteration) and provenance (i.e., geographical origin) of cosmetic grade argan oil collected from different regions of Morocco in 2017. The fatty acid profiles obtained by gas chromatography (GC) showed that oleic acid (C18:1) is the most abundant fatty acid, followed by linoleic acid (C18:2) and palmitic acid (C16:0). The content of trans-oleic and trans-linoleic isomers was between 0.02% and 0.03%, while trans-linolenic isomers were between 0.06% and 0.09%. Discriminant analysis (DA) and orthogonal projection to latent structure—discriminant analysis (OPLS-DA) were performed to discriminate between argan oils from Essaouira, Taroudant, Tiznit, Chtouka-Aït Baha and Sidi Ifni. -

A Technical Glance on Some Cosmetic Oils
European Scientific Journal June 2014 /SPECIAL/ edition vol.2 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 A TECHNICAL GLANCE ON SOME COSMETİC OİLS Kenan Yildirim, PhD Department of Fiber and Polymer Engineering Faculty of Natural Sciences, Architecture and Engineering,Bursa Technical University Bursa, Turkiye A. Melek Kostem, MSc Tübitak-Butal Bursa Test and Analysis Laboratory, Bursa Turkiye Abstract The properties of molecular structure, thermal behavior and UVA protection of 10 types of healthy promoting oils which are argania, almond, apricot seed, and jojoba, wheat germ, and sesame seed, avocado, cocoa, carrot and grapes seed oils were studied. FT-IR analysis was used for molecular structure. DSC analysis was used for thermal behavior and UV analysis was used for UV, visible and IR light absorption. Molecular structure and thermal behavior of avocado and cocoa are different from the others. Contrary to the spectra of avocado and cocoa on which the peaks belongs to carboxylic acid very strong, the spectra of the others do not involve carboxylic acid strong peaks. Almond, wheat germ and apricot seed oil absorb all UVA before 350 nm wave length. The UV protection property of grapes seed and cocoa is very well. Protection of wheat germ, almond and apricot seed oils to UVA is well respectively. Absorption of IR rate change from 7% to 25% for carrot, apricot seed, wheat germ, argania, avocado and grapes seed respectively. Keywords: Cosmetic oils, thermal behavior, molecular structure, UV protection Practical applications Except avocado and cocoa oils the others may be used for production of inherently UV protection yarn. This type of yarn can be used for producing UV protection clothes. -
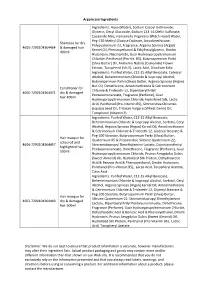
Arganicare Ingredients Disclaimer: We Do Our Best to Ensure That Product
Arganicare Ingredients Ingredients: Aqua (Water), Sodium Cocoyl Isethionate, Glycerin, Decyl Glucoside, Sodium C14-16 Olefin Sulfonate, Cocamide Mea, Hamamelis Virginiana (Witch Hazel) Water, Peg-120 Methyl Glucose Dioleate, Amodimethicone, Shampoo for dry Polyquaternium-22, Fragrance, Argania Spinosa (Argan) 4605-7290104364464 & damaged hair Kernel Oil, Phenoxyethanol & Ethylhexylglycerin, Dmdm 400ml Hydantoin, Niacinamide, Guar Hydroxypropyltrimonium Chloride, Panthenol (Pro-Vit. B5), Butyrospermum Parkii (Shea Butter) Oil, Anthemis Nobilis (Camomile) Flower Extract, Tocopherol (Vit.E), Lactic Acid, Disodium Edta Ingredients: Purifed Water, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Behentrimonium Chloride & Isopropyl Alcohol, Butyrospermum Parkii (Shea) Butter, Argania Spinosa (Argan) Nut Oil, Dimethicone, Amodimethicone & Cetrimonium Conditioner for Chloride & Trideceth-12, Dipentaerythrityl 4606-7290104364471 dry & damaged Pentaisononanoate, Fragrance (Perfume), Guar hair 400ml Hydroxypropyltrimonium Chloride, Hydrolized Silk, Lactic Acid, Panthenol (Pro-Vitamin B5), Simmondsia Chinensis (Jojoba) Seed Oil, Triticum Vulgare (Wheat Germ) Oil, Tocopherol (Vitamin E) Ingredients: Purifed Water, C12-15 Alkyl Benzoate, Behentrimonium Chloride & Isopropyl Alcohol, Sorbitol, Cetyl Alcohol, Argania Spinosa (Argan) Kernel Oil, Amodimethicone & Cetrimonium Chloride & Trideceth-12, Glyceryl Stearate & Peg-100 Stearate, Butyrospermum Parkii (Shea) Butter, Hair masque for Quaternium-95 & Propanediol, Silicone Quaternium-22, coloured and 4604-7290104364457 -

Physicochemical Characterization and Evaluation of Castor Oil (R
American Journal of Applied Chemistry 2019; 7(4): 110-115 http://www.sciencepublishinggroup.com/j/ajac doi: 10.11648/j.ajac.20190704.11 ISSN: 2330-8753 (Print); ISSN: 2330-8745 (Online) Physicochemical Characterization and Evaluation of Castor Oil (R. communis ) for Hair Biocosmetics Solomon Sime Tessema Department of Chemistry, Arba Minch University, Arba Minch, Ethiopia Email address: To cite this article: Solomon Sime Tessema. Physicochemical Characterization and Evaluation of Castor Oil (R. communis ) for Hair Biocosmetics. American Journal of Applied Chemistry. Vol. 7, No. 4, 2019, pp. 110-115. doi: 10.11648/j.ajac.20190704.11 Received : January 26, 2019; Accepted : July 17, 2019; Published : July 30, 2019 Abstract: This study reports the characterization of oil from Castor (Ricinus Communius L) seed oil. The biocosmetic potential of the castor oil was evaluated for hair through physico-chemical characterization. The various physicochemical parameters (iodine value, pH value, specific gravity, refractive index, peroxide value, etc) were tested in accordance with American standard testing method specifications and compared with argan oil. Accordingly, the parameters tested comply with some journals dealing with cosmetics. Biocosmetic has high potential as a raw material for synthetic cosmetics or blend stock substitution for cosmetics without any modification. The advantage of castor oil over other oils (sunflower, olive, soy bean, corn) would lie in the oil price. Keywords: Caster Oil, Castor Beans, Biocosmetic, Nonedible Oil, Soxhlet Extractor, Argan Oil During the last century, synthetic substitutes have become 1. Introduction available and have been used to replace natural seed oils. History tells us that, a long time ago, plants and plant Due to toxic effects of synthetic oils, there is a growing trend products have been used as the primary source of food, to replace them and revert to the use of natural oils in the shelter and transport materials, clothing, fragrances, flavors cosmetic and pharmaceutical industries [6]. -

Carrier Oils
SWEET ALMOND OIL 100% Pure Almond Oil is a natural oil that’s perfect for nourishing and reviving any skin type. Almond Oil is easily absorbed and won’t clog pores, promoting clear, soft, healthy- looking skin. This natural skin-nourishing oil is ideal for the entire body. Almond Oil is a natural oil derived from pressed almonds. Work several drops between your palms and massage into the desired area. For the face, after cleansing, massage 3-5 drops of Organic Almond Oil into your skin, paying particular attention to the area around your eyes. An Ideal carrier oil for essential oil applications. ARGAN OIL Organic Argan oil is cold-pressed from the nut. It is easily absorbed and is perfect for topical applications. It is rich in fatty acid content. Argan helps moisturize, soothe, add shine, and nourish when applied to skin, hair, or nail cuticles. It is rich in vitamin A and E. Again oil can reduce inflammation and assist other beneficial ingredients penetrate the skin and will not clog the pores. Historically was used as a wound treatment and a rash healer. Simply warm a few drops in your hands and massage into your face and neck. For hair a drop or less may do! Great Carrier oil. CASTOR OIL Castor Oil is expeller-pressed from the seed of Ricinus communis and is virtually odorless. While its use is applicable to many other areas of wellness such as detox and promoting lymphatic drainage, castor oil is considered by many to be one of the finest natural emollients available today. -
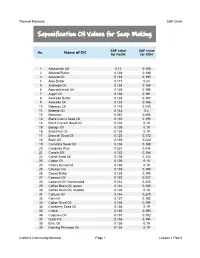
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

Emerging Vegetable Oils in Europe
CBI Product Factsheet: Emerging vegetable oils in Europe CBI | Market Intelligence Product Factsheet Cloves in Germany | 1 Introduction The drive for innovation in the European food industry leads companies to adopt new ingredients in order to remain competitive. The introduction of new vegetable oils (emerging oils), either as ingredients or as final consumer products, is a form of innovation, differentiation and marketing used by food companies to articulate their competitive edge. On the other side of the coin, exporters of emerging oils still face a number of challenges to access the European market successfully. Whereas the Novel Food Regulation imposes marketing restrictions on a number of innovative products, some barriers to suppliers are often related to issues such as supply sufficiency and stability. At the same time, emerging oils also provide opportunities in terms of niche marketing and value adding propositions. Understanding the European market for emerging vegetable oils Innovation is high on the agenda of food manufacturers in Europe, significantly in North-Western Europe. In order to remain competitive, companies invest heavily in Research & Development (R&D), with the goal of adapting products to ever-changing consumer preferences, food health and safety requirements and introducing novelty into the food market. A large part of this strategy consists in finding new ingredient solutions. Vegetable oils are an integral part of several food products and, as such, are an interesting basis for product development. The introduction of new vegetable oils, either as ingredients or as final consumer products, is a form of innovation, differentiation and marketing used by food companies to articulate their competitive edge. -

The Argan Oil Project: Going from Utopia to Reality in 20 Years
OCL 2018, 25(2), D209 © Z. Charrouf and D. Guillaume, Published by EDP Sciences, 2018 https://doi.org/10.1051/ocl/2018006 OCL Oilseeds & fats Crops and Lipids Topical issue on: Available online at: OIL & PROTEIN CROPS: DIFFERENTIATION THROUGH A QUALITY APPROACH www.ocl-journal.org OLÉOPROTÉAGINEUX : SE DÉMARQUER PAR UNE DÉMARCHE QUALITÉ REVIEW The argan oil project: going from utopia to reality in 20 years Zoubida Charrouf1,* and Dominique Guillaume2 1 Laboratoire de Chimie des Plantes et de Synthèse Organique et Bioorganique, Faculté des Sciences, Université Mohammed V, BP 1014, Rabat, Morocco 2 CNRS-UMR7312, UFR Med-Pharm., Chimie Thérapeutique, 51 rue Cognacq-Jay, 51100 Reims, France Received 9 November 2017 – Accepted 22 January 2018 Topical Issue Abstract – The “arganoil project”is nowadays considered as an economic success that harmoniously combined sustainable development, integrated research-action and socio-economic progress. Actually, it was a long battle whose main stages are presented here. The main stages of the argan oil project include a detailed chemical study of argan oil in order to certify argan oil quality and establish an official quality norm and obtain a geographic indication, pharmacological analyses to certify cosmetic argan oil safety, and finally a strong desire to develop Moroccan rural areas by implementing women’s cooperatives and easing the women’s access to education. Keywords: argan oil / edible oil / cosmetic oil / Morocco / field research Résumé – Le projet arganier : 20 ans pour passer de l’utopie à la réalité.. Le « projet arganier » est considéré comme un succès économique qui a mélangé harmonieusement le développement durable, la recherche-action intégrée et le progrès économique et social.