Effects of Fishing on Long-Lived Marine Organisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FEBRUARY 1, 2018 10 11 VOTING MEMBERS 12 Kevin Anson (Designee for Scott Bannon)
1 GULF OF MEXICO FISHERY MANAGEMENT COUNCIL 2 3 268TH MEETING 4 5 FULL COUNCIL SESSION 6 7 Hyatt Centric New Orleans, Louisiana 8 9 JANUARY 29-FEBRUARY 1, 2018 10 11 VOTING MEMBERS 12 Kevin Anson (designee for Scott Bannon)...................Alabama 13 Patrick Banks...........................................Louisiana 14 Leann Bosarge.........................................Mississippi 15 Doug Boyd...................................................Texas 16 Roy Crabtree.................................................NMFS 17 Dale Diaz.............................................Mississippi 18 Phil Dyskow...............................................Florida 19 Tom Frazer................................................Florida 20 Johnny Greene.............................................Alabama 21 Martha Guyas (designee for Jessica McCawley)..............Florida 22 Campo Matens............................................Louisiana 23 Paul Mickle (designee for Joe Spraggins)..............Mississippi 24 Robin Riechers..............................................Texas 25 John Sanchez..............................................Florida 26 Bob Shipp.................................................Alabama 27 Greg Stunz..................................................Texas 28 Ed Swindell.............................................Louisiana 29 30 NON-VOTING MEMBERS 31 Glenn Constant..............................................USFWS 32 Dave Donaldson..............................................GSMFC 33 LT Mark Zanowicz.............................................USCG -

2021 Louisiana Recreational Fishing Regulations
2021 LOUISIANA RECREATIONAL FISHING REGULATIONS www.wlf.louisiana.gov 1 Get a GEICO quote for your boat and, in just 15 minutes, you’ll know how much you could be saving. If you like what you hear, you can buy your policy right on the spot. Then let us do the rest while you enjoy your free time with peace of mind. geico.com/boat | 1-800-865-4846 Some discounts, coverages, payment plans, and features are not available in all states, in all GEICO companies, or in all situations. Boat and PWC coverages are underwritten by GEICO Marine Insurance Company. In the state of CA, program provided through Boat Association Insurance Services, license #0H87086. GEICO is a registered service mark of Government Employees Insurance Company, Washington, DC 20076; a Berkshire Hathaway Inc. subsidiary. © 2020 GEICO CONTENTS 6. LICENSING 9. DEFINITIONS DON’T 11. GENERAL FISHING INFORMATION General Regulations.............................................11 Saltwater/Freshwater Line...................................12 LITTER 13. FRESHWATER FISHING SPORTSMEN ARE REMINDED TO: General Information.............................................13 • Clean out truck beds and refrain from throwing Freshwater State Creel & Size Limits....................16 cigarette butts or other trash out of the car or watercraft. 18. SALTWATER FISHING • Carry a trash bag in your car or boat. General Information.............................................18 • Securely cover trash containers to prevent Saltwater State Creel & Size Limits.......................21 animals from spreading litter. 26. OTHER RECREATIONAL ACTIVITIES Call the state’s “Litterbug Hotline” to report any Recreational Shrimping........................................26 potential littering violations including dumpsites Recreational Oystering.........................................27 and littering in public. Those convicted of littering Recreational Crabbing..........................................28 Recreational Crawfishing......................................29 face hefty fines and litter abatement work. -
MAN CCRC..Ani~::,,"'
,,"!>< MANCCRC..ANi~::,,"',,- ~ ~ r Zjfi>~ ~ 92 DEFINING FISHERIES A User's Glossary Kenneth J. Roberts Jerald W. Horst John E. Roussel JosephA. Shephard LOUISIANA SEA GRANT COLLFGF. ['ROGRAM Louisiana State Vniversity INTRODUCTION Theharvest of fish by commercialand recreationaltishermen is regulatedby federal LOUISIANA andstate agencies that develop plans by which SEA GRANT to managefishery resources.In so doing, College Program agenciesseek advice from fishermen and others involvedwith the fishing industry, Manage- ment plans include technical words and terms thatare difficult to understand,This glossary ~ LauISIAeA givesdefinitions that should help fishermen COOPERATIVE EXTENSION SERVICE understandthe scientific basis of management. EOIIISIENESTREE UNIVERSITT EGRIClj'LTUREI CENTER Thus, their comments and advice will be more usefulin planningand management. Kenneth J. Roberts, Putting fishery managementterms in Marine ResourceEconomics Specialist Louisiana Cooperative Extension Service nonscientificwords may makesome definitions Louisiana State University toosimple, but theauthors sought to getthe main idea acrosson each term. Some terms are Jerald W. Horst, Area Agent Fisheries! referredto by scientistsas letters or groupsof Louisiana Cooperative Extension Service letters acronymsland theseare listed at the end Louisiana StateUniversity of the publicationfor quick reference.The word fish is usedthroughout, and includesfinfish and John E, Roussel, Marine Fisheries Division shellfishspecies, A mail-inform is providedto Louisiana Department of Wildlife and Fisheries identify words and terms that need to be includedin a futureprinting. JosephA, Shephard, FisheriesResearch Division Louisiana Department of Wildlife and Fisheries Amberjack on front cover courtesy of Dr. Bruce A Thompson, LSU Coastal Fisheries Institute The researchthat resulted in this publication was A Seeanrll<al mortality. supported by the Louisiana SeaGrant College ABC Seeallourable biologicai catch. Program of Louisiana State University. -

Chapter 220-3 Marine Resources Division
Conservation and Natural Resources Chapter 220-3 ALABAMA DEPARTMENT OF CONSERVATION AND NATURAL RESOURCES ADMINISTRATIVE CODE CHAPTER 220-3 MARINE RESOURCES DIVISION TABLE OF CONTENTS 220-3-.01 Shrimping 220-3-.02 Oystering 220-3-.03 Use Of Nets And Harvest Of Mullet 220-3-.04 Miscellaneous And Public Access Regulation 220-3-.05ER Harvesting Limit For Oysters (Emergency Rule Expired) 220-3-.06.02ER Discarding Dead Fish And Other Seafoods Into The Gulf Of Mexico 220-3-.07.03ER Closed Shrimping In The Waters Of The Gulf Of Mexico (Emergency Rule Expired) 220-3-.08 Redfish And Speckled Sea Trout Regulations (Repealed 8/10/15) 220-3-.09.07ER Closed Fishing Of Any Speckled Trout (Emergency Rule - Repealed) 220-3-.10ER Discarding Of Dead Fish And Other Dead Seafoods Into The Gulf Of Mexico (Emergency Rule - Expired) 220-3-.11 Red Drum (Redfish) And Spotted Sea Trout Regulations (Repealed 8/10/15) 220-3-.12 Red Drum And Spotted Sea Trout Game Fish Regulation 220-3-.13 (Reserved) 220-3-.14 (Reserved) 220-3-.15 Oyster Season And Harvest Limits 220-3-.16 (Reserved) 220-3-.17ER Prohibition Of Nets - Gulf Of Mexico (Emergency Rule Expired) 220-3-.18 (Reserved) 220-3-.19 (Reserved) 220-3-.20 Prohibition Of Commercial Taking Or Possession, Or Purchase/Sale/Trade Of King Mackerel (Repealed 6/19/18) 220-3-.21ER Prohibition Of Commercial Taking, Possession, Or Landing Of Red Snapper (Emergency Rule Effective 4/6/92) Supp. 9/30/21 3-1 Chapter 220-3 Conservation and Natural Resources 220-3-.22 Creel/Possession Limit On Ling (Cobia) (Repealed 4/20/15) 220-3-.23 -

ASMFC Stock Status Overview
American Eel ASMFC Stock Status Overview American Lobster Atlantic Cobia This document provides an overview of stock status for the Commission’s 27 managed species or species groups. Graphs contain the most recent information available and have been vetted through the relevant species technical committee. Where Atlantic Croaker biomass data is lacking, other fishery indicators are used (i.e., landings, fishing mortality rates). Time frames differ based on Atlantic Herring data availability. October 2020 Atlantic Menhaden Atlantic Striped Bass Atlantic Sturgeon Black Drum Black Sea Bass Bluefish Coastal Sharks Horseshoe Crab Jonah Crab Northern Shrimp Red Drum Scup Shad & River Herring Spanish Mackerel Spiny Dogfish Spot Spotted Seatrout Summer Flounder Tautog Weakfish Vision: Sustainable and Cooperative Management of Atlantic Coastal Fisheries Winter Flounder Quick Guide to ASMFC Species Stock Status (Current as of October 2020) = Rebuilt/Sustainable / = Recovering/Rebuilding = Depleted ? = Unknown *= Concern STATUS/ REBUILDING STATUS & SPECIES OVERFISHED OVERFISHING TRENDS SCHEDULE 2017 stock assessment update American Eel Depleted Unknown indicates resource remains depleted. GOM/GBK stock abundance Gulf of Maine/ has increased since the 1980s. American Georges Bank Not Depleted N 2020 benchmark assessment to Lobster (GOM/GBK) be presented to the Board in October. SNE stock has collapsed and is experiencing recruitment Southern New Depleted N failure. 2020 benchmark England assessment to be presented to the Board in October. Depleted on coastwide basis; Amendment 3 established 2013 moratorium unless river- American Shad Depleted Unknown specific sustainability can be documented; benchmark assessment scheduled for 2020. In 2020, the TLA was updated to incorporate additional fishery-independent surveys, Atlantic age and length information, an Unknown Unknown ? Croaker updated reference period, regional characteristics, and an updated management trigger mechanism. -

Upper Red Lake 04-0035-01 BELTRAMI COUNTY
Upper Red Lake 04-0035-01 BELTRAMI COUNTY Lake Water Quality Summary Upper Red Lake is located to the west of Waskish, MN in Beltrami County. The western half of the basin is located within the Red Lake Reservation. Upper and Lower Red Lake together is the largest inland water body in Minnesota. Upper Red Lake covers 119,274 acres and has a maximum depth of 15 feet (Table 1). Upper Red Lake has 41 inlets and one outlet, which classify it as a drainage lake (Figure 1). Water exits Upper Red Lake to Lower Red Lake and enters the Red Lake River, which flows west to the Red River. Water quality data have been collected on Upper Red Lake since 1990 (Tables 2-3). These data show that the lake is eutrophic (59), which is characteristic of dense aquatic plant growth and algae blooms in late summer. The Upper Red Lake walleye fishery is one of the most valuable resources to the local and state economy of all the lakes in Minnesota. Upper Red Lake is monitored extensively by the Red Lake Department of Natural Resources. This long-term data set is beneficial in future planning for this economically and ecologically valuable water resource. The Upper Red Lake Area Association is active in outreach, tourism promotion, and area events. Its mission is “To promote and enhance the Upper Red Lake Area, and the economic, commercial, and social interested of its inhabitants.” Table 1. Location data and physical characteristics for Upper Red Lake. Location Data Physical Characteristics MN Lake ID: 04-0035-01 Surface area (acres): 119,274 County: Beltrami % Littoral area: 100% Ecoregion: Northern Minnesota Wetlands Max depth (ft), (m): 15, 4.6 Major Drainage Basin: Upper/Lower Red Lake Inlets: 41 Latitude/Longitude: 48.131237 / -94.766452 Outlets: 1 Invasive Species: Starry stonewart Public Accesses: 2 Table 2. -
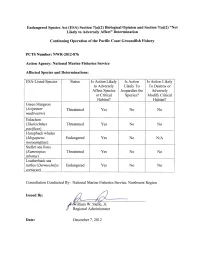
Endangered Species Act (ESA) Section 7(A)(2) Biological Opinion and Section 7(A)(2) "Not Likely to Adversely Affect" Determination
Endangered Species Act (ESA) Section 7(a)(2) Biological Opinion and Section 7(a)(2) "Not Likely to Adversely Affect" Determination Continuing Operation of the Pacific Coast Groundfish Fishery PeTS Number: NWR-2012-876 Action Agency: National Marine Fisheries Service Affected Species and Determinations: ESA-Listed Species Status Is Action Likely Is Action Is Action Likely to Adversely Likely To To Destroy or Affect Species Jeopardize the Adversely or Critical Species? Modify Critical Habitat? Habitat? Green Sturgeon (Acipenser Threatened Yes No No medirostris) Eulachon (Thaleichthys Threatened Yes No No vaci/icus) Humpback whales (Megaptera Endangered Yes No N/A novaeangliae) Steller sea lions (Eumetopias Threatened Yes No No jubatus) Leatherback sea turtles (Dermochelys Endangered Yes No No coriacea) Consultation Conducted By: National Marine Fisheries Service, Northwest Region Issued By: illiam W. Stelle, Jr. Regional Administrator Date: December 7,2012 List of Acronyms ACL Annual Catch Limits AKR Alaska Region A-SHOP At-Sea Hake Observer Program BRT Biological Review Team BRD Bycatch Reduction Device CA/OR/WA California/Oregon/Washington CDFG California Department of Fish and Game CI Confidence Interval COSEWIC Committee on the Status for Endangered Wildlife in Canada CPFVs Commercial Passenger Fishing Vessels CPUE Catch Per Unit Effort DPS Distinct Population Segment DQA Data Quality Act DU Designatable Unit EEZ Exclusive Economic Zone EFH Essential Fishing Habitat EFPs Exempt Fishing Permits ESA Endangered Species Act ESU Evolutionarily -

Florida Recreational Saltwater Fishing Regulations
Florida Recreational Issued: July 2020 New regulations are highlighted in red Saltwater Fishing Regulations (please visit: MyFWC.com/Fishing/Saltwater/Recreational Regulations apply to state waters of the Gulf and Atlantic for the most current regulations) All art: © Diane Rome Peebles, except snowy grouper (Duane Raver) Reef Fish Snapper General Snapper Regulations: • Snapper Aggregate Bag Limit - Within state waters ul of the Atlantic and Gulf, Snapper, Cubera u l Snapper, Red u l X Snapper, Vermilion X Snapper, Lane u l all species of snapper are Minimum Size Limits: Minimum Size Limits: Minimum Size Limits: Minimum Size Limits: included in a 10 fish per • Atlantic and Gulf - 12" (see below) • Atlantic - 20" • Atlantic - 12" • Atlantic and Gulf - 8" harvester per day aggregate • Gulf - 16" • Gulf - 10" bag limit in any combination Daily Recreational Bag Limit: Daily Recreational Bag Limit: of snapper species, unless • Atlantic and Gulf - 10 per harvester Season: Daily Recreational Bag Limit: • Atlantic - 10 per harvester stated otherwise. under 30", included within snapper • Atlantic - Open year-round • Atlantic - 5 per harvester not included • Gulf - 100 pounds per harvester, not • Seasons – If no seasonal aggregate bag limit • Gulf - Open June 11–July 25 within snapper aggregate bag limit included within snapper aggregate • May additionally harvest up to 2 over • Gulf - 10 per harvester not included bag limit information is provided, the Daily Recreational Bag Limit: species is open year-round. 30" per harvester or vessel-whichever within snapper aggregate bag limit is less-, and these 2 fish over 30" are • Atlantic and Gulf - 2 per harvester not included within snapper aggregate • Gulf - Zero daily bag and possession limit bag limit for captain and crew on for-hire vessels. -

2011 Major Rule Changes
2021 MAJOR RULE CHANGES ALL $40 Adult and $30 Junior Entry include entry into Mystery Fish Drawing. What is the Mystery Fish Drawing? $5.00 of adult and the junior entry fee is used to pay randomly drawn prize winners for any Walleye, Saugeye, or Sauger over 15” that is registered. “Any fish can win.” Rain or Shine, the tournament will go on unless river navigation on the river is closed. You must keep your copy of the weigh-in slip to be turned in for the top 40 payouts. This year Pool 7 has been added. To register fish caught in pool 7 participants will need to lock through Dresbach Dam and proceed to register at the Municipal Landing 1700 Marcou Dr. La Crosse (Pool 8-La Crosse) weigh-in site. Lock and dam phone numbers and how to lock through Dresbach are on the back of this Major Rule Changes page. All fish must be transported to weigh-ins by boat in the water only. No trailered boat registration allowed. Pool 9 regulations has a protected slot limit of 20-27 inches for Walleye. Walleye within this slot limit are to be released immediately Weigh-ins will be located at the Municipal Landing 1700 Marcou Dr. La Crosse (Pool 8-La Crosse), Stoddard Public Landing (Pool 8 – Stoddard) and the Dairyland Landing (Pool 9 – Genoa). Pool 7 Fishermen will have to lock through Dam #7 (507)895-2170, to register a fish at the Municipal Landing stated above. The Stoddard weigh-in site will be closed at 12’ River Stage. -

Iowa Fishing Regulations
www.iowadnr.gov/fishing 1 Contents What’s New? Be a Responsible Angler .....................................3 • Mississippi River walleye length limit License & Permit Requirements ..........................3 changes - length limits in Mississippi Threatened & Endangered Species ....................4 River Pools 12-20 now include the entire Health Benefits of Eating Fish .............................4 Mississippi River in Iowa (p. 12). General Fishing Regulations ...............................5 • Missouri River paddlefish season start Fishing Seasons & Limits ....................................9 date changed to Feb. 1 (p. 11) Fish Identification...............................................14 • Virtual fishing tournaments added to License Agreements with Bordering States .......16 Iowa DNR special events applications Health Advisories for Eating Fish.......................17 - the definition of fishing tournaments now Aquatic Invasive Species...................................18 includes virtual fishing tournaments (p. 6) Fisheries Offices Phone Numbers .....................20 First Fish & Master Angler Awards ....................21 Conservation Officers Phone Numbers .............23 License and Permit Fees License/Permit Resident Nonresident On Sale Dec. 15, 2020 On Sale Jan. 1, 2021 Annual 16 years old and older $22.00 $48.00 3-Year $62.00 Not Available 7-Day $15.50 $37.50 3-Day Not Available $20.50 1-Day $10.50 $12.00 Annual Third Line Fishing Permit $14.00 $14.00 Trout Fee $14.50 $17.50 Lifetime (65 years old and older) $61.50 Not Available Boundary Water Sport Trotline $26.00 $49.50 Fishing Tournament Permit $25.00 $25.00 Fishing, Hunting, Habitat Fee Combo $55.00 Not Available Paddlefish Fishing License & Tag $25.50 $49.00 Give your kids a lifetime of BIG memories The COVID-19 pandemic ignited Iowans’ pent-up passion to get out and enjoy the outdoors. -

Vi Fishery-At-A-Glance: White Sturgeon Scientific Name
Fishery-at-a-Glance: White Sturgeon Scientific Name: Acipenser transmontanus Range: In saltwater, White Sturgeon range from Ensenada, Mexico northward to the Gulf of Alaska, but tend to spend much of their time in freshwater and estuarine habitats. Habitat: White Sturgeon primarily inhabit estuaries of large rivers. Size (length and weight): White Sturgeon can grow to be greater than 6 meters (20 feet) long and 630 kilograms (1,389 pounds). Life span: The maximum age is unknown, but White Sturgeon may live to be greater than 100 years. Reproduction: White Sturgeon move upstream to lay their eggs in coarse substrates, and reproduction only occurs in large rivers such as the Sacramento River. Females spawn every 2 to 5 years after reaching 12 to 16 years-old, while males spawn every 1 to 2 years after reaching 10 to 12 years-old. In the Sacramento and San Joaquin river systems, spawning success appears related to high outflows during spring. Prey: Young White Sturgeon feed on small crustaceans and aquatic insects. Diet broadens with growth to include a variety of bottom dwelling invertebrates, including primarily clams, crabs and shrimps, but also fish and fish eggs in certain circumstances (e.g., Eulachon, sculpins, lamprey, and spawning Pacific Herring). Predators: Sculpins, Walleye, Smallmouth Bass and Chinook Salmon are known to prey on eggs and juvenile White Sturgeon less than 1 year-old. Sea Lions and some shark species have been observed to prey upon older juvenile and adult White Sturgeon. The armored bony scutes along the body of juveniles and adults probably reduce predation relative to earlier life stages. -

IPHC Blue Book
International Pacifi c Halibut Commission Eighty-ninth Annual Meeting Meeting Information Navigating the 2013 IPHC Annual Meeting ..................................................................................2 Detailed Meeting Agenda ..............................................................................................................3 Halibut Terminology – What You May Hear .................................................................................6 Staff presentations The Pacifi c halibut fi shery, 2012 ....................................................................................................9 Heather L. Gilroy IPHC Management Strategy Evaluation framework ...................................................................28 Steven Martell Assessment of the Pacifi c halibut stock at the end of 2012 .........................................................32 Ian J. Stewart, Bruce M. Leaman, Steven Martell, and Raymond A. Webster IPHC Staff harvest advice and regulatory proposals: 2013 .......................................................114 Bruce M. Leaman, Ian J. Stewart, Raymond A. Webster, and Heather L. Gilroy Optimal harvest rates for Pacifi c halibut ....................................................................................131 Steven Martell, Ian J. Stewart, and Bruce M. Leaman Coastwide comparison of alternative setline survey baits .........................................................154 Raymond A. Webster, Steven M. Kaimmer, Claude L. Dykstra and Bruce M. Leaman Halibut size at age research