A Clinical-Based Diagnostic Approach to Cerebellar Atrophy in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inherited Cerebellar Ataxia in Childhood: a Pattern-Recognition Approach Using Brain MRI
REVIEW ARTICLE Inherited Cerebellar Ataxia in Childhood: A Pattern-Recognition Approach Using Brain MRI L. Vedolin, G. Gonzalez, C.F. Souza, C. Lourenc¸o, and A.J. Barkovich ABSTRACT SUMMARY: Ataxia is the principal symptom of many common neurologic diseases in childhood. Ataxias caused by dysfunction of the cerebellum occur in acute, intermittent, and progressive disorders. Most of the chronic progressive processes are secondary to degen- erative and metabolic diseases. In addition, congenital malformation of the midbrain and hindbrain can also be present, with posterior fossa symptoms related to ataxia. Brain MR imaging is the most accurate imaging technique to investigate these patients, and imaging abnormalities include size, shape, and/or signal of the brain stem and/or cerebellum. Supratentorial and cord lesions are also common. This review will discuss a pattern-recognition approach to inherited cerebellar ataxia in childhood. The purpose is to provide a comprehensive discussion that ultimately could help neuroradiologists better manage this important topic in pediatric neurology. ABBREVIATIONS: AR ϭ autosomal recessive; CAC ϭ cerebellar ataxia in childhood; 4H ϭ hypomyelination with hypogonadotropic hypogonadism and hypodon- tia; JSRD ϭ Joubert syndrome and related disorders; OPHN1 ϭ oligophrenin-1 taxia is an inability to coordinate voluntary muscle move- plastic/paraneoplastic disorders, immune-mediated/demyelinat- Aments that cannot be attributed to weakness or involuntary ing disorders, and drugs/toxins (antiepileptic medications, -

Probable Olivopontocerebellar Degeneration)
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.34.1.14 on 1 February 1971. Downloaded from J. Neurol. Neurosurg. Psychiat., 1971, 34, 14-19 L-Dopa in Parkinsonism associated with cerebellar dysfunction (probable olivopontocerebellar degeneration) HAROLD L. KLAWANS, JR. AND EARL ZEITLIN From the Department of Neurology, Rush Presbyterian-St. Luke's Medical Center, Chicago, Illinois 60612, USA SUMMARY Two patients with combined cerebellar and Parkinsonian features consistent with olivopontocerebellar degeneration were treated with long term oral L-dopa. Both patients showed improvement of the Parkinsonian symptoms but the cerebellar symptoms were unchanged. It is suggested that the Parkinsonian manifestations of this syndrome are related to loss of dopamine in the striatum secondary to lesions of the substantia nigra. It is suggested that other patients with be a trial with similar disorders should given L-dopa. guest. Protected by copyright. Olivopontocerebellar degeneration was first defined familial form of degeneration with onset between by Dejerine and Thomas (1900). They described the ages of 20 to 30, while they had described a two patients who developed progressive cerebellar sporadic form with onset at a later age. Since the dysfunction. In both instances the onset was in original description of this disease, other investi- middle age, beginning in the legs and later involving gators have described hereditary forms of olivo- the arms. The post-mortem examination of the pontocerebellar degeneration. Keiller (1926) brains or these patients revealed atrophy of the described four cases of olivopontocerebellar de- cerebellar cortex, bulbar olives, and pontine gray generation who had positive hereditary histories. matter with degeneration of the middle and inferior Three of these cases were verified on post-mortem cerebellar peduncles. -
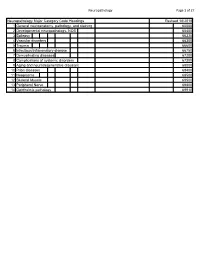
Neuropathology Category Code List
Neuropathology Page 1 of 27 Neuropathology Major Category Code Headings Revised 10/2018 1 General neuroanatomy, pathology, and staining 65000 2 Developmental neuropathology, NOS 65400 3 Epilepsy 66230 4 Vascular disorders 66300 5 Trauma 66600 6 Infectious/inflammatory disease 66750 7 Demyelinating diseases 67200 8 Complications of systemic disorders 67300 9 Aging and neurodegenerative diseases 68000 10 Prion diseases 68400 11 Neoplasms 68500 12 Skeletal Muscle 69500 13 Peripheral Nerve 69800 14 Ophthalmic pathology 69910 Neuropathology Page 2 of 27 Neuropathology 1 General neuroanatomy, pathology, and staining 65000 A Neuroanatomy, NOS 65010 1 Neocortex 65011 2 White matter 65012 3 Entorhinal cortex/hippocampus 65013 4 Deep (basal) nuclei 65014 5 Brain stem 65015 6 Cerebellum 65016 7 Spinal cord 65017 8 Pituitary 65018 9 Pineal 65019 10 Tracts 65020 11 Vascular supply 65021 12 Notochord 65022 B Cell types 65030 1 Neurons 65031 2 Astrocytes 65032 3 Oligodendroglia 65033 4 Ependyma 65034 5 Microglia and mononuclear cells 65035 6 Choroid plexus 65036 7 Meninges 65037 8 Blood vessels 65038 C Cerebrospinal fluid 65045 D Pathologic responses in neurons and axons 65050 1 Axonal degeneration/spheroid/reaction 65051 2 Central chromatolysis 65052 3 Tract degeneration 65053 4 Swollen/ballooned neurons 65054 5 Trans-synaptic neuronal degeneration 65055 6 Olivary hypertrophy 65056 7 Acute ischemic (hypoxic) cell change 65057 8 Apoptosis 65058 9 Protein aggregation 65059 10 Protein degradation/ubiquitin pathway 65060 E Neuronal nuclear inclusions 65100 -

GENETIC TESTING REQUISITION Please Ship All
GENETIC TESTING REQUISITION 1-844-363-4357· [email protected] Schillingallee 68 · 18057 Rostock Germany Attention Patient: Please visit your nearest LifeLabs or CML Healthcare Patient Service Centre for sample collection LL: K012-01/ CML: CEN CONTRACT # Report to Physician Billing # LifeLabs Demographic Ordering Physician Name Label Physician Signature: Ordering Physician Address: Tel: Fax: Address & Contact Info: Copy to (name & contact info): Name: Contact: Bill to Contract # K012-01 (patient does not pay at time of collection) Patient Gender: (M/F) Patient Name (Last, First): Patient DOB: (YYYY/MM/DD) Patient Address: Patient Health Card: Patient Telephone: Please ship all NON-PRENATAL samples to: LifeLabs · Attn CDS Department • 100 International Boulevard• Toronto ON• M9W6J6 TEST REQUESTED LL TR # / CML TC# □ Genetic Test - Blood Sample 2 x 4mL EDTA 4005 □ Genetic Test (Pediatric) - Blood Sample 1 x 2mL EDTA 4008 □ Genetic Test - Other Sample Type 4014 PRENATAL SAMPLES: Please ship directly to CENTOGENE. Date Blood Collected (YYYY/MM/DD): ___________ Time Blood Collected (HH:MM)) :________ Collector Name: ___________________ GENETIC TESTING CONSENT I understand that a DNA specimen will be sent to LifeLabs for genetic testing. My physician has told me about the condition(s) being tested and its genetic basis. I am aware that correct information about the relationships between my family members is important. I agree that my specimen and personal health information may be sent to Centogene AG at their lab in Germany (address below). To ensure accurate testing, I agree that the results of any genetic testing that I have had previously completed by Centogene AG may be shared with LifeLabs. -

Atypical Development of the Cerebellum : Impact on Language Function Lynette M
University of New Mexico UNM Digital Repository Psychology ETDs Electronic Theses and Dissertations 8-28-2012 Atypical development of the cerebellum : impact on language function Lynette M. Silva Follow this and additional works at: https://digitalrepository.unm.edu/psy_etds Recommended Citation Silva, Lynette M.. "Atypical development of the cerebellum : impact on language function." (2012). https://digitalrepository.unm.edu/psy_etds/129 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Psychology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Lynette M. Silva Candidate Psychology Department This dissertation is approved, and it is acceptable in quality and form for publication: Approved by the Dissertation Committee: Steven Verney, Co-Chairperson Ron Yeo, Co-Chairperson Robert Thoma Jean Lowe ATYPICAL DEVELOPMENT OF THE CEREBELLUM: IMPACT ON LANGUAGE FUNCTION By LYNETTE M. SILVA B.A., English, Stanford University, 1996 M.S., Psychology, University of New Mexico, 2009 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Psychology The University of New Mexico Albuquerque, New Mexico July 2012 iii Dedication For my parents, extended family, and valued friends, because it took a village. And for Martin Rodriguez, who served as my Virgil, and showed me the way. iv Acknowledgements I would like to thank Drs. Steven Verney, Ron Yeo, Robert Thoma, and Jean Lowe for their guidance and support as members of my dissertation committee. I am very thankful for the encouragement and supervision I continue to receive from Dr. -

Comprehensive Systematic Review: Treatment of Cerebellar Motor Dysfunction and Ataxia
Comprehensive systematic review: Treatment of cerebellar motor dysfunction and ataxia Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Theresa A. Zesiewicz, MD1; George Wilmot, MD2; Sheng-Han Kuo, MD3; Susan Perlman, MD4; Patricia E. Greenstein, MB, BCh5; Sarah H. Ying, MD6; Tetsuo Ashizawa, MD7; S.H. Subramony, MD8; Jeremy D. Schmahmann, MD9; K.P. Figueroa10; Hidehiro Mizusawa, MD11; Ludger Schöls, MD12; Jessica D. Shaw, MPH1; Richard M. Dubinsky, MD, MPH13; Melissa J. Armstrong, MD, MSc8; Gary S. Gronseth, MD13; Kelly L. Sullivan, PhD14 1) Department of Neurology, University of South Florida, Tampa 2) Department of Neurology, Emory University, Atlanta, GA 3) Department of Neurology, Columbia University, New York, NY 4) Department of Neurology, University of California, Los Angeles 5) Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA 6) Shire, Lexington, MA, and the Johns Hopkins University School of Medicine, Baltimore, MD 7) Department of Neurology, Houston Methodist Research Institute, TX 8) Department of Neurology, University of Florida College of Medicine, Gainesville 9) Department of Neurology, Massachusetts General Hospital, and Department of Neurology, Harvard Medical School, Boston, MA 10) Department of Neurology, University of Utah, Salt Lake City 11) National Center of Neurology and Psychiatry, Tokyo, Japan 12) Department of Neurology and Hertie-Institute for Clinical Brain Research, Tübingen, Germany 13) Department of Neurology, University of Kansas Medical Center, Kansas City 14) Jiann-Ping Hsu College of Public Health, Georgia Southern University, Statesboro Address correspondence and reprint requests to American Academy of Neurology: [email protected] Title character count: 71 Abstract word count: 254 Manuscript word count: 7,891 Approved by the Guideline Development, Dissemination, and Implementation Subcommittee on October 22, 2016; by the Practice Committee on October 2, 2017; and by the AAN Institute Board of Directors on December 5, 2017. -

Arthrogryposis and Congenital Myasthenic Syndrome Precision Panel
Arthrogryposis and Congenital Myasthenic Syndrome Precision Panel Overview Arthrogryposis or arthrogryposis multiplex congenita (AMC) is a group of nonprogressive conditions characterized by multiple joint contractures found throughout the body at birth. It usually appears as a feature of other neuromuscular conditions or part of systemic diseases. Primary cases may present prenatally with decreased fetal movements associated with joint contractures as well as brain abnormalities, decreased muscle bulk and polyhydramnios whereas secondary causes may present with isolated contractures. Congenital Myasthenic Syndromes (CMS) are a clinically and genetically heterogeneous group of disorders characterized by impaired neuromuscular transmission. Clinically they usually present with abnormal fatigability upon exertion, transient weakness of extra-ocular, facial, bulbar, truncal or limb muscles. Severity ranges from mild, phasic weakness, to disabling permanent weakness with respiratory difficulties and ultimately death. The mode of inheritance of these diseases typically follows and autosomal recessive pattern, although dominant forms can be seen. The Igenomix Arthrogryposis and Congenital Myasthenic Syndrome Precision Panel can be as a tool for an accurate diagnosis ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved, and their high or intermediate penetrance. -

Early ACCESS Diagnosed Conditions List
Iowa Early ACCESS Diagnosed Conditions Eligibility List List adapted with permission from Early Intervention Colorado To search for a specific word type "Ctrl F" to use the "Find" function. Is this diagnosis automatically eligible for Early Medical Diagnosis Name Other Names for the Diagnosis and Additional Diagnosis Information ACCESS? 6q terminal deletion syndrome Yes Achondrogenesis I Parenti-Fraccaro Yes Achondrogenesis II Langer-Saldino Yes Schinzel Acrocallosal syndrome; ACLS; ACS; Hallux duplication, postaxial polydactyly, and absence of the corpus Acrocallosal syndrome, Schinzel Type callosum Yes Acrodysplasia; Arkless-Graham syndrome; Maroteaux-Malamut syndrome; Nasal hypoplasia-peripheral dysostosis-intellectual disability syndrome; Peripheral dysostosis-nasal hypoplasia-intellectual disability (PNM) Acrodysostosis syndrome Yes ALD; AMN; X-ALD; Addison disease and cerebral sclerosis; Adrenomyeloneuropathy; Siemerling-creutzfeldt disease; Bronze schilder disease; Schilder disease; Melanodermic Leukodystrophy; sudanophilic leukodystrophy; Adrenoleukodystrophy Pelizaeus-Merzbacher disease Yes Agenesis of Corpus Callosum Absence of the corpus callosum; Hypogenesis of the corpus callosum; Dysplastic corpus callosum Yes Agenesis of Corpus Callosum and Chorioretinal Abnormality; Agenesis of Corpus Callosum With Chorioretinitis Abnormality; Agenesis of Corpus Callosum With Infantile Spasms And Ocular Anomalies; Chorioretinal Anomalies Aicardi syndrome with Agenesis Yes Alexander Disease Yes Allan Herndon syndrome Allan-Herndon-Dudley -

Cerebellar-Lesions.Pdf
Cerebellar Lesions Author: Lisa Heusel-Gillig, PT, DPT, NCS Fact Sheet Many individuals present to emergency rooms with acute symptoms of vertigo and imbalance. Others seek consultation from physicians with gradual imbalance and dizziness. In either case, determining the presence of cerebellar involvement with or without peripheral vestibular hypofunction has important treatment implications. Cerebellar or Brainstem Stroke Patients presenting to the emergency room with vertigo and imbalance should be tested for a cerebellar or brainstem stroke. However, a lesion may not always be apparent on a CT scan. If the history and other clinical findings are not consistent with benign paroxysmal positional vertigo (BPPV), peripheral vestibular neuritis, or vestibular migraine, a stroke should be considered. One way to differentiate between a stroke and a peripheral problem is the inability of the individual to Produced by coordinate his legs to walk. Anterior Inferior Cerebellar Artery (AICA) Stroke If the cerebellar stroke is related to a blockage or hemorrhage of the anterior inferior cerebellar artery (AICA), there is a possibility that the labyrinthine artery could be affected. The labyrinthine artery supplies the peripheral vestibular apparatus. In this case, patients would also have both hearing loss and peripheral vestibular hypofunction on the same side of the stroke. These patients should be A Special Interest referred to a clinic that specializes in both vestibular function testing and treating Group of patients with central and peripheral vestibular involvement. Cerebellar Atrophy or Degeneration Cerebellar degeneration is a progressive disease, which presents with an ataxic gait and imbalance.1 Subtypes may also affect both central and peripheral pathways and cause abnormalities in the vestibular ocular reflex (VOR) as well as oculomotor deficits.2,3 Studies have shown there is a high risk of falls with injuries with this 4 Contact us: population and fall prevention therapy is strongly suggested. -

DEMENTIA in Cerebellar Volume in Genetic FTD
RESEARCH HIGHLIGHTS Nature Reviews Neurology | Published online 11 Mar 2016; doi:10.1038/nrneurol.2016.28 DEMENTIA in cerebellar volume in genetic FTD. “We decided to investigate the volume of cerebellar subregions to Cerebellar atrophy has determine whether specific areas are associated with mutations in the key FTD genes,” explains Rohrer. disease-specific patterns Using MRI, the team determined the volumes of cerebellar subregions Distinct patterns of cerebellar question that remained was whether in 44 patients with mutations in atrophy relate to wider patterns of cerebellar changes were associated C9orf72, MAPT or GRN. GRN Patterns of disease-specific brain network degen- with cortical changes, or just occurred mutations were not associated with cerebellar eration, according to two recent concomitantly,” says Hornberger. cerebellar atrophy, whereas C9orf72 atrophy studies. The findings reveal details The team used MRI to visu- mutations were specifically associated of cerebellar atrophy in Alzheimer alize cerebellar degeneration in with atrophy in lobule VIIa–Crus I, differed disease (AD) and frontotemporal 217 patients with AD or one of and MAPT mutations were specif- between AD dementia (FTD), with implications three FTD subtypes: behavioural ically associated with atrophy in and bvFTD for future research and therapy. variant FTD, nonfluent variant pri- the vermis. Cerebellar degeneration has mary progressive aphasia (nfvPPA), “C9orf72‑associated atrophy largely been disregarded in demen- and semantic variant PPA (svPPA). reflects degeneration of a cortico- tia owing to its association with They then compared the atrophy thalamo-cerebellar network impor- movement disorders. However, the maps with an atlas of cerebral and tant in cognition, and the vermis cerebellum is involved in cognition cerebellar connectivity. -

VLDLR-Associated Cerebellar Hypoplasia
VLDLR-associated cerebellar hypoplasia Description VLDLR-associated cerebellar hypoplasia is an inherited condition that affects the development of the brain. People with this condition have an unusually small and underdeveloped cerebellum, which is the part of the brain that coordinates movement. This brain malformation leads to problems with balance and coordination (ataxia) that become apparent in infancy and remain stable over time. Children with VLDLR- associated cerebellar hypoplasia may learn to walk later in childhood, usually after the age of 6, although some are never able to walk independently. In one Turkish family, affected people walk on their hands and feet (quadrupedal locomotion). Additional features of VLDLR-associated cerebellar hypoplasia include moderate to profound intellectual disability, impaired speech (dysarthria) or a lack of speech, and eyes that do not look in the same direction (strabismus). Some affected individuals have also had flat feet (pes planus), seizures, and short stature. Studies suggest that VLDLR- associated cerebellar hypoplasia does not significantly affect a person's life expectancy. Frequency VLDLR-associated cerebellar hypoplasia is rare; its prevalence is unknown. The condition was first described in the Hutterite population in Canada and the United States. This condition has also been reported in families from Iran and Turkey. Causes As its name suggests, VLDLR-associated cerebellar hypoplasia results from mutations in the VLDLR gene. This gene provides instructions for making a protein called a very low density lipoprotein (VLDL) receptor. Starting before birth, this protein plays a critical role in guiding the movement of developing nerve cells to their appropriate locations in the brain. Mutations in the VLDLR gene prevent cells from producing any functional VLDL receptor protein. -

Anti-GAD Antibodies and Periodic Alternating Nystagmus
OBSERVATION Anti-GAD Antibodies and Periodic Alternating Nystagmus Caroline Tilikete, MD; Alain Vighetto, MD; Paul Trouillas, MD; Jérome Honnorat, MD Background: Autoantibodies directed against glu- Intervention: Baclofen, a GABAergic medication, was tamic acid decarboxylase (GAD-Ab) have recently been given to the patient. described in a few patients with progressive cerebellar ataxia, suggesting an autoimmune physiopathologic Main Outcome Measures: Eye movement recording mechanism. of spontaneous nystagmus and postrotatory vestibular responses. Objective: To determine the exact role of GAD-Ab and ␥-aminobutyric acid (GABA)–ergic neurotransmission in Results: Baclofen was effective in suppressing PAN and the pathogenesis of cerebellar ataxia. improving postrotatory vestibular responses but not for improving cerebellar ataxia. Design: Case report. Conclusion: The presence of PAN and the response to Setting: University neurological hospital. baclofen provide a unique opportunity to suggest a direct role of GAD-Ab in cerebellar dysfunction in this Patient: We report the case of a patient with subacute patient. cerebellar ataxia associated with GAD-Ab showing pe- riodic alternating nystagmus (PAN). Arch Neurol. 2005;62:1300-1303 LUTAMIC ACID DECARBOX- ebellar ataxia associated with periodic al- ylase (GAD) is a major ternating nystagmus (PAN). Precise enzyme of the nervous knowledge of the physiopathologic mecha- system that catalyzes the nism of this nystagmus and its response conversion of glutamate to treatment may help to better under-