GENETIC TESTING REQUISITION Please Ship All
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inherited Cerebellar Ataxia in Childhood: a Pattern-Recognition Approach Using Brain MRI
REVIEW ARTICLE Inherited Cerebellar Ataxia in Childhood: A Pattern-Recognition Approach Using Brain MRI L. Vedolin, G. Gonzalez, C.F. Souza, C. Lourenc¸o, and A.J. Barkovich ABSTRACT SUMMARY: Ataxia is the principal symptom of many common neurologic diseases in childhood. Ataxias caused by dysfunction of the cerebellum occur in acute, intermittent, and progressive disorders. Most of the chronic progressive processes are secondary to degen- erative and metabolic diseases. In addition, congenital malformation of the midbrain and hindbrain can also be present, with posterior fossa symptoms related to ataxia. Brain MR imaging is the most accurate imaging technique to investigate these patients, and imaging abnormalities include size, shape, and/or signal of the brain stem and/or cerebellum. Supratentorial and cord lesions are also common. This review will discuss a pattern-recognition approach to inherited cerebellar ataxia in childhood. The purpose is to provide a comprehensive discussion that ultimately could help neuroradiologists better manage this important topic in pediatric neurology. ABBREVIATIONS: AR ϭ autosomal recessive; CAC ϭ cerebellar ataxia in childhood; 4H ϭ hypomyelination with hypogonadotropic hypogonadism and hypodon- tia; JSRD ϭ Joubert syndrome and related disorders; OPHN1 ϭ oligophrenin-1 taxia is an inability to coordinate voluntary muscle move- plastic/paraneoplastic disorders, immune-mediated/demyelinat- Aments that cannot be attributed to weakness or involuntary ing disorders, and drugs/toxins (antiepileptic medications, -

CRASH Syndrome: Clinical Spectrum Vance Lemmon0 Guy Van Campa of Corpus Callosum Hypoplasia, Lieve Vitsa Retardation, Adducted Thumbs, Paul Couckea Patrick J
Review 1608178 Eur J Hum Genet 1995;3:273-284 Erik Fransen3' CRASH Syndrome: Clinical Spectrum Vance Lemmon0 Guy Van Campa of Corpus Callosum Hypoplasia, Lieve Vitsa Retardation, Adducted Thumbs, Paul Couckea Patrick J. Willemsa Spastic Paraparesis and a Department of Medical Genetics, Hydrocephalus Due to Mutations in University of Antwerp, Belgium; One Single Gene, L1 b Case Western Reserve University, Cleveland, Ohio, USA Keywords Abstract X-linked disorder LI is a neuronal cell adhesion molecule with important func Mental retardation tions in the development of the nervous system. The gene Hydrocephalus encoding LI is located near the telomere of the long arm of the MASA syndrome X chromosome in Xq28. We review here the evidence that Adducted thumbs several X-linked mental retardation syndromes including X- Corpus callosum agenesis linked hydrocephalus (HSAS), MASA syndrome, X-linked Spastic paraplegia complicated spastic paraparesis (SPI) and X-linked corpus LI callosum agenesis (ACC) are all due to mutations in the LI CRASH gene. The inter- and intrafamilial variability in families with Mutation analysis an LI mutation is very wide, and patients with HSAS, MASA, SPI and ACC can be present within the same family. There fore, we propose here to refer to this clinical syndrome with the acronym CRASH, for Corpus callosum hypoplasia, Retar dation, Adducted thumbs, Spastic paraplegia and Hydroceph alus. Clinical Aspects for Hydrocephalus due to Stenosis of the Aqueduct of Sylvius. This designation was X-Linked Hydrocephalus based upon the presence of aqueductal steno X-linked hydrocephalus (MIM No. sis in many HSAS patients [1]. However, later 307000) was originally described by Bickers studies reported several HSAS patients with and Adams in 1949. -

Podocyte Specific Knockdown of Klf15 in Podocin-Cre Klf15flox/Flox Mice Was Confirmed
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1: Podocyte specific knockdown of Klf15 in Podocin-Cre Klf15flox/flox mice was confirmed. (A) Primary glomerular epithelial cells (PGECs) were isolated from 12-week old Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice and cultured at 37°C for 1 week. Real-time PCR was performed for Nephrin, Podocin, Synaptopodin, and Wt1 mRNA expression (n=6, ***p<0.001, Mann-Whitney test). (B) Real- time PCR was performed for Klf15 mRNA expression (n=6, *p<0.05, Mann-Whitney test). (C) Protein was also extracted and western blot analysis for Klf15 was performed. The representative blot of three independent experiments is shown in the top panel. The bottom panel shows the quantification of Klf15 by densitometry (n=3, *p<0.05, Mann-Whitney test). (D) Immunofluorescence staining for Klf15 and Wt1 was performed in 12-week old Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice. Representative images from four mice in each group are shown in the left panel (X 20). Arrows show colocalization of Klf15 and Wt1. Arrowheads show a lack of colocalization. Asterisk demonstrates nonspecific Wt1 staining. “R” represents autofluorescence from RBCs. In the right panel, a total of 30 glomeruli were selected in each mouse and quantification of Klf15 staining in the podocytes was determined by the ratio of Klf15+ and Wt1+ cells to Wt1+ cells (n=6 mice, **p<0.01, unpaired t test). Supplementary Figure 2: LPS treated Podocin-Cre Klf15flox/flox mice exhibit a lack of recovery in proteinaceous casts and tubular dilatation after DEX administration. -

Seq2pathway Vignette
seq2pathway Vignette Bin Wang, Xinan Holly Yang, Arjun Kinstlick May 19, 2021 Contents 1 Abstract 1 2 Package Installation 2 3 runseq2pathway 2 4 Two main functions 3 4.1 seq2gene . .3 4.1.1 seq2gene flowchart . .3 4.1.2 runseq2gene inputs/parameters . .5 4.1.3 runseq2gene outputs . .8 4.2 gene2pathway . 10 4.2.1 gene2pathway flowchart . 11 4.2.2 gene2pathway test inputs/parameters . 11 4.2.3 gene2pathway test outputs . 12 5 Examples 13 5.1 ChIP-seq data analysis . 13 5.1.1 Map ChIP-seq enriched peaks to genes using runseq2gene .................... 13 5.1.2 Discover enriched GO terms using gene2pathway_test with gene scores . 15 5.1.3 Discover enriched GO terms using Fisher's Exact test without gene scores . 17 5.1.4 Add description for genes . 20 5.2 RNA-seq data analysis . 20 6 R environment session 23 1 Abstract Seq2pathway is a novel computational tool to analyze functional gene-sets (including signaling pathways) using variable next-generation sequencing data[1]. Integral to this tool are the \seq2gene" and \gene2pathway" components in series that infer a quantitative pathway-level profile for each sample. The seq2gene function assigns phenotype-associated significance of genomic regions to gene-level scores, where the significance could be p-values of SNPs or point mutations, protein-binding affinity, or transcriptional expression level. The seq2gene function has the feasibility to assign non-exon regions to a range of neighboring genes besides the nearest one, thus facilitating the study of functional non-coding elements[2]. Then the gene2pathway summarizes gene-level measurements to pathway-level scores, comparing the quantity of significance for gene members within a pathway with those outside a pathway. -

Hereditary Spastic Paraparesis: a Review of New Developments
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.69.2.150 on 1 August 2000. Downloaded from 150 J Neurol Neurosurg Psychiatry 2000;69:150–160 REVIEW Hereditary spastic paraparesis: a review of new developments CJ McDermott, K White, K Bushby, PJ Shaw Hereditary spastic paraparesis (HSP) or the reditary spastic paraparesis will no doubt Strümpell-Lorrain syndrome is the name given provide a more useful and relevant classifi- to a heterogeneous group of inherited disorders cation. in which the main clinical feature is progressive lower limb spasticity. Before the advent of Epidemiology molecular genetic studies into these disorders, The prevalence of HSP varies in diVerent several classifications had been proposed, studies. Such variation is probably due to a based on the mode of inheritance, the age of combination of diVering diagnostic criteria, onset of symptoms, and the presence or other- variable epidemiological methodology, and wise of additional clinical features. Families geographical factors. Some studies in which with autosomal dominant, autosomal recessive, similar criteria and methods were employed and X-linked inheritance have been described. found the prevalance of HSP/100 000 to be 2.7 in Molise Italy, 4.3 in Valle d’Aosta Italy, and 10–12 Historical aspects 2.0 in Portugal. These studies employed the In 1880 Strümpell published what is consid- diagnostic criteria suggested by Harding and ered to be the first clear description of HSP.He utilised all health institutions and various reported a family in which two brothers were health care professionals in ascertaining cases aVected by spastic paraplegia. The father was from the specific region. -

MASA Syndrome in Twin Brothers: Case Report of Sixteen-Year Clinical Follow Up
Paediatr Croat. 2014;58:286-90 PRIKAZ BOLESNIKA / CASE REPORT www.paedcro.com http://dx.doi.org/10.13112/PC.2014.50 MASA syndrome in twin brothers: case report of sixteen-year clinical follow up Matilda Kovač Šižgorić1, Zlatko Sabol1, Filip Sabol2, Tonći Grmoja3, Svjetlana Bela Klancir1, Zdravka Gjergja1, Ljiljana Kipke Sabol1 MASA syndrome (OMIM 303350) is a rare X-linked recessive neurologic disorder, also called CRASH syndrome, spastic paraplegia 1 and Gareis-Mason syndrome. The acronym MASA describes four major signs: Mental retardation, Aphasia, Shuffl ing gait and Adducted thumbs. A more suitable name for this syndrome is L1 syndrome because the disorder has been associated with mutations in the neuronal cell adhesion molecule L1 (L1CAM) gene. The syndrome has severe symptoms in males, while females are carriers because only one X chromosome is aff ected. The aim of this report is to show similarities and diff erences in clinical manifestations between twins with the L1CAM gene mutation and to emphasize the importance of genetic counseling. Our patients were dizygotic twins born prematurely at 35 weeks of gestation. Pregnancy was complicated with early bleeding and gestational diabetes. Immediately after birth, hypertonia of lower extremities was observed in both twins. Sixteen-year clinical follow up showed spastic paraparetic form with shuffl ing gait, clumsiness, delayed speech development, lower intellectual functioning at the level of mild to moderate mental retarda- tion, primary nocturnal enuresis, behavioral and sleep disorder (more pronounced in the second twin). Magnetic resonance imaging of the brain showed complete agenesis of the corpus callosum, complete lack of the anterior commissure, and internal hydrocephalus. -

AP-4 Mediates Export of ATG9A from the Trans-Golgi Network to Promote
AP-4 mediates export of ATG9A from the trans-Golgi PNAS PLUS network to promote autophagosome formation Rafael Matteraa,1, Sang Yoon Parka,1, Raffaella De Pacea, Carlos M. Guardiaa, and Juan S. Bonifacinoa,2 aCell Biology and Neurobiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892 Edited by Pietro De Camilli, Howard Hughes Medical Institute and Yale University, New Haven, CT, and approved November 6, 2017 (received for review October 2, 2017) AP-4 is a member of the heterotetrameric adaptor protein (AP) mediated by a noncanonical YRYRF sequence in the receptor- complex family involved in protein sorting in the endomembrane associated, transmembrane AMPA receptor regulatory proteins system of eukaryotic cells. Interest in AP-4 has recently risen with (TARPs) (17). Finally, in the δ2 glutamate receptor protein, the discovery that mutations in any of its four subunits cause a binding to μ4 depends on several phenylalanine residues in a form of hereditary spastic paraplegia (HSP) with intellectual noncanonical context (18). disability. The critical sorting events mediated by AP-4 and the Interest in AP-4 has recently risen because of the discovery of pathogenesis of AP-4 deficiency, however, remain poorly under- mutations in genes encoding each of the subunits of AP-4 in stood. Here we report the identification of ATG9A, the only a subset of autosomal recessive hereditary spastic paraplegias multispanning membrane component of the core autophagy ma- (HSPs), namely, SPG47 (AP4B1/β4), SPG50 (AP4M1/μ4), SPG51 chinery, as a specific AP-4 cargo. AP-4 promotes signal-mediated (AP4E1/e), and SPG52 (AP4S1/σ4) (19–21). -
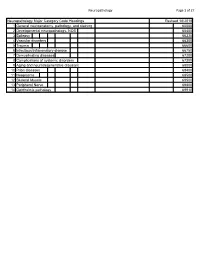
Neuropathology Category Code List
Neuropathology Page 1 of 27 Neuropathology Major Category Code Headings Revised 10/2018 1 General neuroanatomy, pathology, and staining 65000 2 Developmental neuropathology, NOS 65400 3 Epilepsy 66230 4 Vascular disorders 66300 5 Trauma 66600 6 Infectious/inflammatory disease 66750 7 Demyelinating diseases 67200 8 Complications of systemic disorders 67300 9 Aging and neurodegenerative diseases 68000 10 Prion diseases 68400 11 Neoplasms 68500 12 Skeletal Muscle 69500 13 Peripheral Nerve 69800 14 Ophthalmic pathology 69910 Neuropathology Page 2 of 27 Neuropathology 1 General neuroanatomy, pathology, and staining 65000 A Neuroanatomy, NOS 65010 1 Neocortex 65011 2 White matter 65012 3 Entorhinal cortex/hippocampus 65013 4 Deep (basal) nuclei 65014 5 Brain stem 65015 6 Cerebellum 65016 7 Spinal cord 65017 8 Pituitary 65018 9 Pineal 65019 10 Tracts 65020 11 Vascular supply 65021 12 Notochord 65022 B Cell types 65030 1 Neurons 65031 2 Astrocytes 65032 3 Oligodendroglia 65033 4 Ependyma 65034 5 Microglia and mononuclear cells 65035 6 Choroid plexus 65036 7 Meninges 65037 8 Blood vessels 65038 C Cerebrospinal fluid 65045 D Pathologic responses in neurons and axons 65050 1 Axonal degeneration/spheroid/reaction 65051 2 Central chromatolysis 65052 3 Tract degeneration 65053 4 Swollen/ballooned neurons 65054 5 Trans-synaptic neuronal degeneration 65055 6 Olivary hypertrophy 65056 7 Acute ischemic (hypoxic) cell change 65057 8 Apoptosis 65058 9 Protein aggregation 65059 10 Protein degradation/ubiquitin pathway 65060 E Neuronal nuclear inclusions 65100 -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Myopathy Genes (HGNC) Neuropathy (HGNC) Neuromuscular Disease
Myopathy Genes Neuropathy Neuromuscular Disease (HGNC) (HGNC) (HGNC) ABHD5 ABCA1 ADCK3 ACTG2 ACO2 AGRN AGK AGXT ALS2 ALDOA AIFM1 ANG AMER1 ALAD AP4B1 ANO5 AMACR AP4E1 AR AP1S1 AP4M1 AUH APTX AP4S1 B4GALT1 AR AP5Z1 CACNA1S ATL3 ATM CASQ1 B4GALNT1 ATXN10 CCDC78 BAG3 ATXN7 CHCHD10 BRP44L BEAN1 CHRNA1 C12orf65 C9orf72 CHRNB1 C19orf12 CACNB4 CHRND C1NH CAPN3 CHRNE CECR1 CHAT CLPB CISD2 CHKB COL6A1 CLCF1 CHMP2B COL6A2 CLCN2 CHRNG COL6A3 CLP1 CLCN1 COLQ CMT2G COL9A3 CTNS CMT2H COQ2 DGUOK CMTDIA COQ6 DNA2 CMTX2 COQ9 DNAJB6 CMTX3 COX15 DNAJC19 COASY CPT1A DNM2 COX6A1 CYP7B1 DPM2 CPOX DAG1 DYSF CYP27A1 DDHD2 EMD CYP2U1 DOK7 EPG5 DARS2 DPAGT1 FAM111B DCAF8 DPM3 FBXL4 DDHD1 DUX4 FKBP14 DFNX5 ECEL1 FKRP DHTKD1 ERBB3 FLH1 DIAPH3 ERLIN2 FLNC DNAJB2 FA2H HNRNPA1 DNAJC3 FKTN HNRNPDL ELOVL5 FUS HNRPA2B1 ERCC8 G6PC KLHL40 FAH GFPT1 KLHL41 FAM126A GLE1 LAMA2 FBN1 GYS2 LDB3 FMR1 HSPD1 LMOD3 FXN IFRD1 MEGF10 GALC INF2 MGME1 GBE1 ISPD MTAP GJC2 ITGA7 MTMR14 GP1BA ITPR1 MYF6 HADHA KCNA1 MYH14 HADHB KCNC3 MYLK2 HFE KCNE3 NARS2 HINT1 KCNJ18 NEB HK1 KCNJ2 ORAI1 HMBS KIAA0196 PRKAG2 HSD17B4 KIF21A PTEN HSN1B L1CAM RBCK1 IARS2 LAMB2 RET IGHMBP2 LARGE RMND1 KCNJ10 MCCC2 SCN4A KIF5A MRE11A SERAC1 LRSAM1 MRPL3 SGCA LYST MTO1 SIL1 MANBA MTPAP SPEG MARS MTTP STAC3 MTATP6 MUSK STIM1 MYH14 MYBPC3 SYNE1 MYOT MYH3 SYNE2 NAMSD MYH8 TAZ NF2 NF1 TIA1 NGLY1 NIPA1 TMEM43 NMSR NOP56 TNPO3 NOTCH3 OPTN TNXB OPA1 PDSS2 TPM2 OPA3 PDYN TRPV4 OTOF PFN1 UBA1 PDK3 PHKA2 VCP PDSS1 PHKG2 XDH PEX10 PHOX2A ACADS PEX2 PIP5K1C ACADVL PMM2 PLEC ACTA1 PNPLA6 PLP1 AGL PPOX POMGNT1 AMPD1 PRICKLE1 -

Mutation in the AP4M1 Gene Provides a Model for Neuroaxonal Injury in Cerebral Palsy
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ARTICLE Mutation in the AP4M1 Gene Provides a Model for Neuroaxonal Injury in Cerebral Palsy Annemieke J.M.H. Verkerk,1 Rachel Schot,2 Belinda Dumee,1 Karlijn Schellekens,1 Sigrid Swagemakers,1 Aida M. Bertoli-Avella,2 Maarten H. Lequin,3 Jeroen Dudink,4 Paul Govaert,4 A.L. van Zwol,4 Jennifer Hirst,5 Marja W. Wessels,2 Coriene Catsman-Berrevoets,6 Frans W. Verheijen,2 Esther de Graaff,2 Irenaeus F.M. de Coo,6 Johan M. Kros,7 Rob Willemsen,2 Patrick J. Willems,8 Peter J. van der Spek,1 and Grazia M.S. Mancini2,* Cerebral palsy due to perinatal injury to cerebral white matter is usually not caused by genetic mutations, but by ischemia and/or inflam- mation. Here, we describe an autosomal-recessive type of tetraplegic cerebral palsy with mental retardation, reduction of cerebral white matter, and atrophy of the cerebellum in an inbred sibship. The phenotype was recorded and evolution followed for over 20 years. Brain lesions were studied by diffusion tensor MR tractography. Homozygosity mapping with SNPs was performed for identification of the chromosomal locus for the disease. In the 14 Mb candidate region on chromosome 7q22, RNA expression profiling was used for selecting among the 203 genes in the area. In postmortem brain tissue available from one patient, histology and immunohistochemistry were performed. Disease course and imaging were mostly remi- niscent of hypoxic-ischemic tetraplegic cerebral palsy, with neuroaxonal degeneration and white matter loss. -

Mackenzie's Mission Gene & Condition List
Mackenzie’s Mission Gene & Condition List What conditions are being screened for in Mackenzie’s Mission? Genetic carrier screening offered through this research study has been carefully developed. It is focused on providing people with information about their chance of having children with a severe genetic condition occurring in childhood. The screening is designed to provide genetic information that is relevant and useful, and to minimise uncertain and unclear information. How the conditions and genes are selected The Mackenzie’s Mission reproductive genetic carrier screen currently includes approximately 1300 genes which are associated with about 750 conditions. The reason there are fewer conditions than genes is that some genetic conditions can be caused by changes in more than one gene. The gene list is reviewed regularly. To select the conditions and genes to be screened, a committee comprised of experts in genetics and screening was established including: clinical geneticists, genetic scientists, a genetic pathologist, genetic counsellors, an ethicist and a parent of a child with a genetic condition. The following criteria were developed and are used to select the genes to be included: • Screening the gene is technically possible using currently available technology • The gene is known to cause a genetic condition • The condition affects people in childhood • The condition has a serious impact on a person’s quality of life and/or is life-limiting o For many of the conditions there is no treatment or the treatment is very burdensome for the child and their family. For some conditions very early diagnosis and treatment can make a difference for the child.