Theme 2 Workshop: Accelerating Therapeutic and Diagnostics Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Name Surname Position Organisation Teleri Lewis Widening Participation
Name Surname Position Organisation Teleri Lewis Widening Participation Manager Aberystwyth University Amy Low Service Delivery Director Abilitynet Helen Wickes Education and Workplace Relationship Manager AbilityNet Michelle Anson Outreach Coordinator AccessHE Geraldine Douglas Uni Connect Outreach Hub Coordinator AccessHE Beth Hayden Outreach Coordinator AccessHE Naz Khan Uni Connect Manager AccessHE Mair Lawrence-Matthews Project Officer AccessHE Tayler Meredith Outreach Coordinator AccessHE Bill Hunt Director of Higher Education Activate Learning Andrew Willis Head of Quality Assurance (HE) Activate Learning Lisa Bates Access and Participation Manager AECC University College Kirsty Allen Community Engagement Project Officer Aimhigher London Jenna Darby UniConnect Hub Officer Aimhigher London Mark Ellis Project Manager Aimhigher London Debra Ibbotson Uni Connect Outreach Hubs Manager Aimhigher London Rory Sheridan Programme Assistant & Disability Directory Project Coordinator Aimhigher London Greg Walker Uni Connect Hub Officer Aimhigher London Baljinder Rana Head of Aimhigher West Midlands Aimhigher West Midlands Emma Thomas Managing Director Applied Inspiration Jessica Woodsford Director for SEER Applied Inspiration Cara Coenen Regional Koordinator for North-Rhine Westfalia/Germany ArbeiterKind.de Amy Knott Outreach & Recruitment Officer Arden University Louise Miller-Marshall Tutor Articulacy Julia Ward Director Articulacy UK Ltd Sarah Dymott Post 16 Education Liaison and Outreach Officer Arts University Bournemouth Sarah Horseman -

Dundee Discovered an Integrated Brand Action Plan
Dundee Discovered An Integrated Brand Action Plan DUNDEE’S LOCAL ACTION PLAN IN THE FRAME OF URBACT- CITYLOGO Aarhus | Alba Iulia | Coimbra | Dundee | Genoa | Oslo | Utrecht | Vilnius | Warsaw | Zaragoza Contents Local Support Group .................................. 1 Are you talking to me? Our key audiences ........................ 19 Introduction Our priorities ................................. 19 Convenor of City Development, Cllr Will Dawson .......................................... 2 Doing better with less Background to Project and Digital and Social Media ................. 20 Local Action Plan Integration of brand and URBACT Programme 2007 -2013 ............ 3 city events ........................................ 21 CityLogo Rationale .................................. 3 Staying current CityLogo – Dundee Baseline .................... 3 Keeping relevant ........................... 22 Dundee’s Brand Development to date Reflecting the changes ................. 22 Background ............................................... 7 What’s the difference? Dundee Narrative ...................................... 9 Connecting Brand development Dundee Ambassadors ............................... 11 and Economic Development ......... 23 Visual Narrative ......................................... 13 What does success look like? ...... 23 Target Audiences ....................................... 15 Tools for measuring ....................... 23 Current Challenges and paths for the near Future Set of Actions ................................... 25 Whose -

Business Bulletin Iris Ghnothaichean
Thursday 10 May 2018 Business Bulletin Iris Ghnothaichean Today's Business Meeting of the Parliament Committee Meetings 11:40 am Parliamentary Bureau Motions 9:00am Culture, Tourism, Europe and 11:40 am General Questions External Relations Committee 12:00 pm First Minister's Questions 9:00am Public Audit and Post-legislative Scrutiny Committee 12:45 pm Members' Business — S5M-11968 Tavish Scott: HIAL's Car Parking Charges 9:00am Public Petitions Committee 2:30 pm Parliamentary Bureau Motions 9:00am Social Security Committee 2:30 pm Scottish Government Debate: A 9:30am Equalities and Human Rights Route Map to an Energy Efficient Scotland Committee followed by Business Motions 1:00pm Justice Sub-Committee on Policing followed by Parliamentary Bureau Motions 1:10pm Culture, Tourism, Europe and External Relations Committee 5:00 pm Decision Time Thursday 10 May 2018 1 Today's Business Future Business Motions & Questions Legislation Other Gnothaichean an-diugh Gnothaichean ri teachd Gluasadan agus Ceistean Reachdas Eile Chamber | Seòmar Meeting of the Parliament 11:40 am Parliamentary Bureau Motions 11:40 am General Questions 1. Ash Denham: To ask the Scottish Government, in light of the ongoing review of the Children (Scotland) Act 1995, what consideration it is giving to putting a professional system, such as the Children and Family Court Advisory and Support Service in England, in place for family courts in Scotland. (S5O-02077) 2. Jamie Halcro Johnston: To ask the Scottish Government what assessment it has made of the effectiveness of the Flexible Workforce Development Fund. (S5O-02078) 3. Bob Doris: To ask the Scottish Government how centres that provide a supervised contact facility for absent parents to spend time with their children are inspected and regulated. -
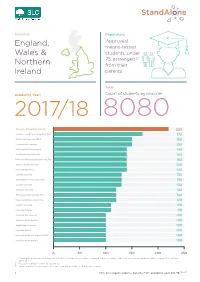
FOI 158-19 Data-Infographic-V2.Indd
Domicile: Population: Approved, England, means-tested Wales & students, under 25, estranged [1] Northern from their Ireland parents Total: Academic Year: Count of students by provider 2017/18 8080 Manchester Metropolitan University 220 Liverpool John Moores University (LJMU) 170 De Montfort University (DMU) 150 Leeds Beckett University 150 University Of Wolverhampton 140 Nottingham Trent University 140 University Of Central Lancashire (UCLAN) 140 Sheeld Hallam University 140 University Of Salford 140 Coventry University 130 Northumbria University Newcastle 130 Teesside University 130 Middlesex University 120 Birmingham City University (BCU) 120 University Of East London (UEL) 120 Kingston University 110 University Of Derby 110 University Of Portsmouth 100 University Of Hertfordshire 100 Anglia Ruskin University 100 University Of Kent 100 University Of West Of England (UWE) 100 University Of Westminster 100 0 50 100 150 200 250 1. “Estranged” means the customer has ticked the “You are irreconcilably estranged (have no contact with) from your parents and this will not change” box on their application. 2. Results rounded to nearest 10 customers 3. Where number of customers is less than 20 at any provider this has been shown as * 1 FOI | Estranged students data by HEP, academic year 201718 [158-19] Plymouth University 90 Bangor University 40 University Of Huddersfield 90 Aberystwyth University 40 University Of Hull 90 Aston University 40 University Of Brighton 90 University Of York 40 Staordshire University 80 Bath Spa University 40 Edge Hill -

REGISTER of STUDENT SPONSORS Date: 27-January-2021
REGISTER OF STUDENT SPONSORS Date: 27-January-2021 Register of Licensed Sponsors This is a list of institutions licensed to sponsor migrants under the Student route of the points-based system. It shows the sponsor's name, their primary location, their sponsor type, the location of any additional centres being operated (including centres which have been recognised by the Home Office as being embedded colleges), the rating of their licence against each route (Student and/or Child Student) they are licensed for, and whether the sponsor is subject to an action plan to help ensure immigration compliance. Legacy sponsors cannot sponsor any new students. For further information about the Student route of the points-based system, please refer to the guidance for sponsors in the Student route on the GOV.UK website. No. of Sponsors Licensed under the Student route: 1,130 Sponsor Name Town/City Sponsor Type Additional Status Route Immigration Locations Compliance Abberley Hall Worcester Independent school Student Sponsor Child Student Abbey College Cambridge Cambridge Independent school Student Sponsor Child Student Student Sponsor Student Abbey College Manchester Manchester Independent school Student Sponsor Child Student Student Sponsor Student Abbotsholme School Uttoxeter Independent school Student Sponsor Child Student Student Sponsor Student Abercorn School London Independent school Student Sponsor Child Student Student Sponsor Student Aberdour School Educational Trust Tadworth Independent school Student Sponsor Child Student Abertay University -

Adult Trade January-June 2018
BLOOMSBURY January – June 2018 NEW TITLES January – June 2018 2 Original Fiction 12 Paperback Fiction 26 Crime, Thriller & Mystery 32 Paperback Crime, Thriller & Mystery 34 Original Non-Fiction 68 Food 78 Wellbeing 83 Popular Science 87 Nature Writing & Outdoors 92 Religion 93 Sport 99 Business 102 Maritime 104 Paperback Non-fiction 128 Bloomsbury Contact List & International Sales 131 Social Media Contacts 132 Index export information TPB Trade Paperback PAPERBACK B format paperback (dimensions 198 mm x 129 mm) Peach Emma Glass Introducing a visionary new literary voice – a novel as poetic as it is playful, as bold as it is strangely beautiful omething has happened to Peach. Blood runs down her legs Sand the scent of charred meat lingers on her flesh. It hurts to walk but she staggers home to parents that don’t seem to notice. They can’t keep their hands off each other and, besides, they have a new infant, sweet and wobbly as a jelly baby. Peach must patch herself up alone so she can go to college and see her boyfriend, Green. But sleeping is hard when she is haunted by the gaping memory of a mouth, and working is hard when burning sausage fat fills her nostrils, and eating is impossible when her stomach is swollen tight as a drum. In this dazzling debut, Emma Glass articulates the unspeakable with breathtaking clarity and verve. Intensely physical, with rhythmic, visceral prose, Peach marks the arrival of a ground- breaking new talent. 11 JANUARY 2018 HARDBACK • 9781408886694 • £12.99 ‘An immensely talented young writer . Her fearlessness renews EBOOK • 9781408886670 • £10.99 one’s faith in the power of literature’ ANZ PUB DATE 01 FEBRUARY 2018 George Saunders HARDBACK • AUS $24.99 • NZ $26.99 TERRITORY: WO ‘You'll be unable to put it down until the very last sentence’ TRANSLATION RIGHTS: BLOOMSBURY Kamila Shamsie ‘Peach is a work of genius. -

Hatred of Capitalism Would Be a Much Better Title
"I looked through your magazine and I was repelled by the title, Semiotext(e). It's so dry, you just want to throw it in the trash, which I did. Listen: Hatred of Capitalism would be a much better title. It's stunning. The world is starving for thoughts. If you can think of something, the language will fall into place, but the thought is what's going to do it." —Jack Smith A READER edited by Chris Kraus and Sylvère Lotringer SEMIOTEXT(E) ACKNOWLEDGEMENTS The editors would like to thank all the friends who helped us retype portions of this manuscript: Priyanka Basu, Shannon Durbin, Jim Fletcher, Giovanni Intra, D'Arcy Cook Jones, John Kelsey, Hedi El Kholti, Tessa Laird, Joan Laughlin, Allison Madigsohn, Keith Pirlot, Sara Reich, Steve Shimada, Tom Simpson, Mark Stritzel, Joel Tauber, John Tremblay, and Robert Hardwick Weston Additional editing: Mark Von Schlegell Assistant editors: Shannon Durbin and Tessa Laird Designed at The Royal Academy of Nuts + Bolts, D.O.D. www.TheRoyalAcademy.org We gratefully acknowledge financial assistance in the publication of this book from the California Arts Council. This work, published as part of a program of aid for publication, received support from the French Ministry of Foreign Affairs and the Cultural Services of the French Embassy in the United States. Cover photo by Mark Borthwick Text at top of back cover from Algeria by Kathy Acker HATRED OF CAPITALISM, A READER. Copyright © 2001 Edited by Chris Kraus and Sylvère Lotringer. All rights reserved. Printed in the United States of America. No part of this book may be used or reproduced in any manner whatsoever without written permission except in the case of brief quotations embodied in critical articles and reviews. -

Snow Patrol ‘Chasing Cars’
Rockschool Grade Pieces Snow Patrol ‘Chasing Cars’ Snow Patrol SONG TITLE: CHASING CARS ALBUM: EYES OPEN RELEASED: 2006 LABEL: POLYDOR GENRE: INDIE PERSONNEL: GARY LIGHTBODY (VOX+GTR) NATHAN CONNOLLY (GTR) PAUL WILSON (BASS) JONNY QUINN (DRUMS) TOM SIMPSON (KEYS) UK CHART PEAK: 6 US CHART PEAK: 5 BACKGROUND INFO NOTES ‘Chasing Cars’ is the second single from Snow Although it didn’t achieve a number 1 in the UK or Patrol’s 2006 album Eyes Open. It is a based on a the U.S. ‘Chasing Cars’ still receives massive airplay single three-chord progression, but ‘Chasing Cars’ is and can be heard almost constantly in TV shows. A far from simple. The song starts with a sparse picked moving acoustic version of ‘Chasing Cars’ appears on eighth-note guitar line which is augmented by subtle the soundtrack for the US TV show Grey’s Anatomy. keyboard parts. The arrangement uses changes in dynamics to develop the song. The third chorus sees ‘Chasing Cars’ move up another notch adding RECOMMENDED LISTENING drums and several distorted guitars playing different inversions (where the notes of a chord are arranged Snow Patrol’s songs are masterpieces of in a different order) to create an orchestra-like wall of arrangement and see the guitar adopting a supporting guitars. The end of the song sees the song return to role on their songs rather than the dominant riffs its sparse beginnings with the re-stating of the simple and extended guitar solos you might expect to hear picked guitar part. from a rock, blues or metal band. -

Top 200 of the Year 2016 #1 Experience with Institution National Universities the Times / the Sunday Times 6 Years in a Row Student Survey 2015 2016
00 / 00 THEBRIDGE THE UNIVERSITY OF DUNDEE ONE OF THE WORLD'S SCOTTISH UNIVERSITY FOR STUDENT BEST IN SCOTLAND FOR SATISFACTION TOP 200 OF THE YEAR 2016 #1 EXPERIENCE WITH INSTITUTION NATIONAL UNIVERSITIES THE TIMES / THE SUNDAY TIMES 6 YEARS IN A ROW STUDENT SURVEY 2015 2016 06 14 16 30 Gary Lightbody interview Dundee: a changing city Love Dundee Projects funded by you Snow Patrol's Gary Lightbody Mike Galloway, Executive We asked for your tales of From sports clubs to scholarships, looks back on life in Dundee Director of City Development love and romance through the cancer research to clinical and where it has led him as he for Dundee City Council, has decades at Dundee. The response practice, alumni have helped receives his honorary degree. been given control of shaping was overwhelming. Read the fund numerous projects across the city in the 21st century. best inside. the University. DACB_Bridge_May2016_new.qxp_DACB_Bridge_May2016 11/05/2016 21:34 Page 1 03 SURVIVAL TIP 1:3 Organising a conference? CONTENTS it makes perfect sense UNIVERSITY OF DUNDEE ALUMNI MAGAZINE Hello from Alumni Relations 05 / PRINCIPAL’S MESSAGE to team up with One of our alumni once said to me that people make Dundee and fine people they are - as both an alumna 06 / GARY LIGHTBODY INTERVIEW and member of staff I couldn’t agree more! This year we have revamped The Bridge so it is very much about the 08 / SCHOLARSHIPS AND BURSARIES people who make Dundee, whether our staff or indeed dundee & angus you, our alumni. From the alumna who has integrated 10 / ART & DESIGN palliative care into the health care system in Kenya to the alumnus who has revolutionised the online shopping 12 / DENTISTRY world, our Dundee ‘folk’ are transforming lives in many different ways. -

SEVENTH SPRING CONFERENCE of the MULTINATIONAL FINANCE SOCIETY (As of April 8, 2019)
SEVENTH SPRING CONFERENCE OF THE MULTINATIONAL FINANCE SOCIETY (As of April 8, 2019) April 19-21, 2019 Civitel Akali Hotel 55 Kissamou str. 731 31 Chania Crete, Greece SESSION 1 Friday 8:30 - 10:30 a.m. ELPIDA ASSET PRICING MODELS Session Chair: Renata Herrerias - ITAM, Mexico "Are Anomalies Exploitable?" Deniz Anginer - World Bank, USA Gerard Hoberg - University of Southern California, USA Hasan Seyhun - University of Michigan, USA Discussant: Ronald Balvers - McMaster University, Canada "Distinguishing Factors and Characteristics with Characteristic-Mimicking Portfolios" Ronald Balvers - McMaster University, Canada Hao Luo - Office of the Comptroller of the Currency, USA Discussant: Georgios Skoulakis - University of British Columbia, Canada "Oil and Equity Return Predictability: The Importance of Dissecting Oil Price Changes" Haibo Jiang - Tulane University, USA Georgios Skoulakis - University of British Columbia, Canada Jinming Xue - University of Maryland, USA Discussant: David Allen - University of Sydney, Australia "“Choosing Factors” by Fama and French (2018): A Comment" David Allen - University of Sydney, Australia Michael McAleer - Asia University, Taiwan Discussant: Hasan Seyhun - University of Michigan, USA SESSION 2 Friday 8:30 - 10:30 a.m. ARIANTHI ACCOUNTING ISSUES Session Chair: Sangwon Suh - Chung-Ang University, Korea, Republic of "Using Google Searches of Firm Products to Assess Revenue Quality and Detect Revenue Management" Peng-Chia Chiu - Chinese University of Hong Kong, Hong Kong Siew Hong Teoh - University of California, -

Snow Patrol Songs for Polarbears Mp3, Flac, Wma
Snow Patrol Songs For Polarbears mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Rock Album: Songs For Polarbears Country: UK Released: 1998 Style: Lo-Fi, Indie Rock MP3 version RAR size: 1673 mb FLAC version RAR size: 1606 mb WMA version RAR size: 1866 mb Rating: 4.1 Votes: 126 Other Formats: DMF MP2 AHX ADX WMA AAC VOX Tracklist Hide Credits 1 Downhill From Here 3:24 Starfighter Pilot 2 3:19 Drums, Keyboards – Richard Colburn 3 The Last Shot Ringing In My Ears 4:26 Absolute Gravity 4 2:46 Scratches [Turntable Tactician] – Tom Simpson 5 Get Balsamic Vinegar...Quick You Fool 3:27 6 Mahogany 2:47 NYC 7 4:28 Vocals – Isobel Campbell 8 Little Hide 2:42 9 Make Up 2:12 10 Velocity Girl 4:37 Days Without Paracetamol 11 3:32 Guitar – Fraser Simpson 12 Fifteen Minutes Old 3:09 13 Favourite Friend 2:46 14 One Hundred Things You Should Have Done In Bed 6:14 Credits Producer – Jamie Watson Barcode and Other Identifiers Barcode: 5 027731 785049 Other versions Category Artist Title (Format) Label Category Country Year Snow Songs For Polarbears (LP, Jeepster JPRLP004 JPRLP004 UK 1998 Patrol Album) Recordings Snow Songs For Polarbears Jeepster JPRCD 004X JPRCD 004X UK 2006 Patrol (CD, Album, S/Edition) Recordings Snow Songs For Polarbears Jeepster NR4039 NR4039 US 1999 Patrol (CD, Album, Promo) Recordings Snow Songs For Polarbears Jeepster JPR 4 JPR 4 US 2006 Patrol (CD, Album, RE) Recordings Ltd. Snow Songs For Polarbears NR4039 Never Records NR4039 US 1999 Patrol (CD, Album) Related Music albums to Songs For Polarbears by Snow Patrol Snow Patrol - Eyes Open Snow Patrol - Chasing Cars / Open Your Eyes (Oomen & Van Doorn Remixes) Snow Patrol - Greatest Hits Snow Patrol - When It's All Over We Still Have To Clear Up Party Patrol - Party Patrol Snow Patrol - Crack The Shutters Snow Patrol - Fallen Empires Snow Patrol - A Hundred Million Suns. -

Scotland Golf Adventure Guide the Essential Planning Resource for Great Scottish Golf Trips
1 Scotland Golf Adventure Guide The essential planning resource for great Scottish golf trips. Contents Introduction Using This Guide (PDF Version) Scotland Tourism Board Scotland Golf Adventure Guide, 1 Planning my first golf trip to Scotland was time This guide is designed to be printed on letter- or Scotland’s National Tourism Board is the official The Big Picture, 2 consuming and difficult. The tourist board informa- A4-size paper, but you can also view it directly in tourism marketing body for Scotland. They main- tion, travel guides, books about golf in Scotland, Adobe Acrobat. It’s your choice. tain an excellent web site at: Tools for Planning Your Trip, 3 and countless web sites overwhelmed me with Printing When to Go, 4 possibilities but provided minimal guidance on www.visitscotland.com In the Adobe Reader toolbar click the Print button making smart choices. And whenFree faced with Demonearly PDF Temperature & Rainfall Statistics, 5 , or choose File > Print. Specify the printer, page Contact them and they will gladly send you a free 600 golf courses and thousands of hotels, you range, number of copies and other options, and Vacation Planner. Be sure to also ask for a copy Building a Rough Itinerary, 6 need to make a lot of choices. This is a FREE DEMO PDFclick of OK. the Enable Scotland (check) Golfthe option to “Shrink of the Official Guide to Golf in Scotland. Use the Sample Itineraries, 7 I started by filteringAdventure the golf course Guide. and accom I -hope youoversized like pages what to paperyou size.”see. web site to request these publications or call: Getting on St Andrews Old & Muirfield, 8 modation choices down to a manageable number.