Error Analysis Example Error Analysis Is Always a Difficult Area for Students
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -
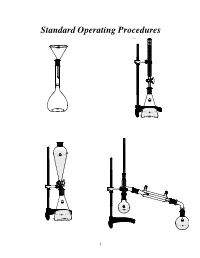
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

A Simple Spectrophotometric Method for the Determination Of
J Clin Pathol: first published as 10.1136/jcp.14.2.202 on 1 March 1961. Downloaded from 202 Technical methods usual laboratory filters may give falsely increased values.) Of the clear and colourless filtrate 10 ml. is pipetted into A a 25 ml. measuring flask and 10 ml. reagent (3) added. simple spectrophotometric After mixing dilute with distilled water to the mark and method for the determination of renew mixing. After 10 minutes the mixture is ready for reading. The colour is stable, according to Watt and urea in blood and urine Chrisp, for 11 days. READING T. K. WITH, TOVE DREYER PETERSEN, AND BIRGIT PETERSEN From the Central Laboratory, Svend- The reactions are read in a spectrophotometer at 420 nm. borg County Hospital, Denmark with a blank prepared in the same way as the reaction mixture but with 3 ml. of distilled water instead of 3 ml. of blood. The urea standard (reagent 4) is treated in the Several methods for urea in determining blood and urine same way as the blood. Cuvettes of 1 cm. thickness are are in use in clinical laboratories, but none is ideal in suitable. mass routine The analysis. spectrophotometric method If p, s, and b are the extinctions of the the based on a modified Ehrlich sample, reagent proposed by Watt standard, and the blank respectively, the urea concen- and Chrisp (1954) for pure solutions is therefore of tration is interest because it can be modified for use in mass clinical analyses of blood and urine. Brown (1959) has l00(p-b)/(s-b) mg./100 ml. -

Environmental Protection Agency Pt. 63, App. A
Environmental Protection Agency Pt. 63, App. A APPENDIX A TO PART 63—TEST METHODS posed as an alternative test method to meet an applicable requirement or in the absence METHOD 301—FIELD VALIDATION OF POLLUT- of a validated method. Additionally, the val- ANT MEASUREMENT METHODS FROM VARIOUS idation procedures of Method 301 are appro- WASTE MEDIA priate for demonstration of the suitability of alternative test methods under 40 CFR parts USING METHOD 301 59, 60, and 61. If, under 40 CFR part 63 or 60, 1.0 What is the purpose of Method 301? you choose to propose a validation method other than Method 301, you must submit and 2.0 What approval must I have to use Method obtain the Administrator’s approval for the 301? candidate validation method. 3.0 What does Method 301 include? 2.0 What approval must I have to use Method 301? 4.0 How do I perform Method 301? If you want to use a candidate test method REFERENCE MATERIALS to meet requirements in a subpart of 40 CFR part 59, 60, 61, 63, or 65, you must also request 5.0 What reference materials must I use? approval to use the candidate test method according to the procedures in Section 16 of SAMPLING PROCEDURES this method and the appropriate section of 6.0 What sampling procedures must I use? the part (§ 59.104, § 59.406, § 60.8(b), § 61.13(h)(1)(ii), § 63.7(f), or § 65.158(a)(2)(iii)). 7.0 How do I ensure sample stability? You must receive the Administrator’s writ- ten approval to use the candidate test meth- DETERMINATION OF BIAS AND PRECISION od before you use the candidate test method to meet the applicable federal requirements. -

I. Direct Titration of Sulfate, II. High Precision Spectrophotometric Analysis Max Quentin Freeland Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1955 I. Direct titration of sulfate, II. High precision spectrophotometric analysis Max Quentin Freeland Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons Recommended Citation Freeland, Max Quentin, "I. Direct titration of sulfate, II. High precision spectrophotometric analysis" (1955). Retrospective Theses and Dissertations. 14746. https://lib.dr.iastate.edu/rtd/14746 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMi films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -

Q & a – Accuracy and Precision
Food Analysis – FScN 4312W Laboratory: Assessment of Accuracy and Precision Key to Questions 1. Theoretically, how are standard deviation, coefficient of variation, mean, percent relative error, and 95% confidence interval affected by: (1) more replicates, and (2) a larger size of measurement? Was this evident in looking at the actual results obtained using the volumetric pipettes and the buret, with n = 3 vs n = 6, and with 1mL vs 10mL? Ans: (1) As the sample size increases (more replicates) - Calculated mean approaches the true mean - Standard deviation is inversely proportional to the square root of sample size, hence it decreases - CV decreases, as standard deviation approaches 0 for larger sample size - % Relative error decreases as calculated mean approaches true mean - 95% confidence interval narrows down (2) Larger size of measurement - Mean is close to true mean - Standard deviation might be larger due to larger size - CV decreases - % Relative error decreases, as calculated mean is close to true mean - 95% confidence interval narrows down Not all of the above assumptions were confirmed in this experiment according to the data observed. One reason could be that the sample size was very small (n = 3 and n = 6), to notice significant differences, another reason could be human error and variation from one person’s measuring technique to another. 2. Why are percent relative error and coefficient of variation used to compare the accuracy and precision, respectively, of the volumes from pipetting/dispensing 1 and 10mL with the volumetric pipettes and buret in parts 2 and 3, rather than simply the mean and standard deviation, respectively? Ans: Mean calculated from the readings gives the calculated mean, which may differ from the true mean. -

SPECIAL SCIENTIFIC REPORT-FISHERIES Na 349
349 CHEMICAL ANALYSES OF MARINE AND ESTUARINE WATERS USED BY THE GALVESTON BIOLOGICAL LABORATORY SPECIAL SCIENTIFIC REPORT-FISHERIES Na 349 UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE United States Department of the Interior, Fred A. Seaton, Secretary Fish and Wildlife Service, Arnie J. Suomela, Commissioner Bureau of Commercial Fisheries, Donald L, McKernan, Director CHEMICAL ANALYSES OF MARINE AND ESTUARINE WATERS USED BY THE GALVESTON BIOLOGICAL LABORATORY by Kenneth T. Marvin, Zoula P. Zein-Eldin, Billie Z. May and Larence M. Lansford Chemists Galveston, Texas United States Fish and Wildlife Service Special Scientific Report— Fisheries No. 349 Washington, D. C. June 1960 CONTENTS Introduction 1 Sample treatment prior to analysis 1 Sample storage containers 2 Analytical methods 2 Standard samples 2 Phosphate 3 Inorganic only 3 Total and inorganic 3 Total only 4 Nitrate-nitrite 5 Nitrite 5 Salinity 6 Copper 6 Sulfide 7 Oxygen 7 Total carbon dioxide 8 Ammonia 10 Chlorophyll 10 "Carbohydrates" 11 "Protein" (tyrosine equivalent) 12 Washing procedure for all analytical glassware 12 Literature cited 13 111 CHEMICAL AMLYSES OF MAP.INE AND ESTOARIKE mTERS USED BY TBE GALVESTON BIOLOGICAL lABORATORY by Kenneth T. Marvin, Zoula P. Zein-Eldin, Billie Z. May and Larence M. Lansford ABSTRACT This paper describes the chemical techniques and procedures used hy the Biological lahoratory of the U. S. Bureau of Commercial Jlsherles, Galveston^ Texas, for analyzing samples Involved In the chemical and hlo- logical survey of the marine and estuarlne waters of the Gulf of Mexico and also In the many laboratory and field studies and experiments that have heen made pertaining to the red tide investigation. -

Laboratory Equipment Used in Filtration
KNOW YOUR LAB EQUIPMENTS Test tube A test tube, also known as a sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Beakers Beakers are used as containers. They are available in a variety of sizes. Although they often possess volume markings, these are only rough estimates of the liquid volume. The markings are not necessarily accurate. Erlenmeyer flask Erlenmeyer flasks are often used as reaction vessels, particularly in titrations. As with beakers, the volume markings should not be considered accurate. Volumetric flask Volumetric flasks are used to measure and store solutions with a high degree of accuracy. These flasks generally possess a marking near the top that indicates the level at which the volume of the liquid is equal to the volume written on the outside of the flask. These devices are often used when solutions containing dissolved solids of known concentration are needed. Graduated cylinder Graduated cylinders are used to transfer liquids with a moderate degree of accuracy. Pipette Pipettes are used for transferring liquids with a fixed volume and quantity of liquid must be known to a high degree of accuracy. Graduated pipette These Pipettes are calibrated in the factory to release the desired quantity of liquid. Disposable pipette Disposable transfer. These Pipettes are made of plastic and are useful for transferring liquids dropwise. Burette Burettes are devices used typically in analytical, quantitative chemistry applications for measuring liquid solution. Differing from a pipette since the sample quantity delivered is changeable, graduated Burettes are used heavily in titration experiments. -

TOP-014, Technical Operating Procedure for Spectrophotometric
CENTER FOR NUCLEAR WASTE Proc. T0P-014 REGULATORY ANALYSES Revision ° TECHNICAL OPERATING PROCEDURE Page I of 11 Title TECHNICAL OPERATING PROCEDURE FOR SPECTROPHOTOMETRIC DETERMINATION OF SILICA EFFECTIVITY AND APPROVAL Revision n of this procedure became effective on 03/04/91 This procedure consists of the pages and changes listed below. Page No. Change Date Effective ALL 03/04/91 Superseaes Procedure No. None Approvals Written By Technical Revie / CENTER FOR NUCLEAR WASTE Proc.TOP-014 REGULATORY ANALYSES Revision _ TECHNICAL OPERATING PROCEDURE Page 2 of 1il TOP-014 TECHNICAL OPERATING PROCEDURE FOR SPECTROPHOTOMETRIC DETERMINATION OF SILICA 1. PURPOSE The purpose of this procedure is to describe a general method for the spectrophotometric determination of silica (Si02) in aqueous solutions. This procedure will be used for aqueous solutions generated by geochemical experiments involving silicate minerals (e.g., zeolites and feldspars) but may also be used for analyses of water samples collected from laboratory experiments or in the field. This procedure implements the requirements of CQAM Section 3. 2. APPLICABLE DOCUMENT The following document forms a part of this procedure, as applicable: Operator's Manual for Milton Roy Spectronic 1201 Spectrophotometer (1988) 3. RESPONSIBILITY (1) The Geosciences Element Manager shall be responsible for the development and maintainance of this procedure. (2) The cognizant Principal Investigator shall be responsible for the implementation of this procedure. (3) Personnel performing tasks described in this procedure are responsible for complying with its requirements. 4. EQUIPMENT (1) Milton Roy Spectronic 1201 Spectrophotometer with microprocessor-controlled functions and features including a linear curve fit test mode or equivalent. -

BLAUBRAND® Volumetric Instruments and Density Bottles
BLAUBRAND® Volumetric Instruments and Density Bottles Testing Instructions (SOP) March 2015 1. Introduction The standard DIN EN ISO 4787 describes both the design and the testing of the volumetric instruments of glass. The following Testing Instructions describe how to apply the ISO standard in practice. We recommend a testing every 1-3 years. The interval depends on the using of aggressive media and the cleaning procedure. These Instructions may also be used as a basis for the supervision of testing devices to DIN EN ISO 9001, DIN EN ISO 10012 and DIN EN ISO/IEC 17025. The test of the Density Bottles was effected on the basis of DIN EN ISO 4787. Meniscus adjustment with BLAUBRAND® Volumetric Instruments Meniscus adjustment Meniscus adjustment with ring mark with Schellbach stripe Read at the lowest point of the meniscus. Read at the point where the two arrows touch. Meniscus adjustment reflexion of liquid surface meniscus ring mark dark paper (p.e. black, blue) 2 2. Preparation for testing 2. Clear identification of the volumetric instrument to be tested Batch number, individual serial number, trademark, nominal volume and tolerances are directly printed ⇒ The test starts with a clear identification of the on every BLAUBRAND volumetric instruments. volumetric instrument in the test record. 2.1 Copy Test Record (see page 13) 2.2 Serial number/Identification number ⇒ Enter into Test Record All BLAUBRAND® volumetric instruments always carry a batch number, e.g., 13.04, or an individual serial number in the case of individual certificates, e.g., 13.040371 (year of production 2013, Batch No. -

List of Equipment
Mfg & Exporters: All types of Turnkey Projects, , Beverages, Mineral Water plant & OEM Spares List Of Equipment SR.NO Equipment Qty 1. Autoclave 1 Vertical Complete Heavy gauze with pressure gauze, Safety Valve, Stream Release Valve Size 30 X 30 cm 2. Digital Hot Air Ageing oven 1 Size 12” x 12” x 12” made of MS powder coated. Make- HB 3. Digital pH System : 1 Make- HB 4. Digital Incubator Bacteriological 2 12” X 12” X 12” made of MS powder coated. Make- HB 5. Water bath double walled 1 inner chamber of SS outer M.S powder coated with 6 hole with thermostatic control Make- HB 6. INNOCULATION CHAMBER, M.S POWDER COATED WITH 1 UV & 1 FLOROCENT LIGHT INBUILT 1 Make- HB 7. Automatic Hot Plate with 8 inch dia with Thermostat 1 Make-HB 8. Digital Spectrophotometer 1 Make- HB 9. Digital Turbidity meter 1 Make- HB 10. Digital Colony Counter 1 Make- HB 11. S.S. Filter holder 47 mm 1 (complete S.S) 12. Microscope student 1 Make-Labron 13. Magnetic Stirrer with Hot Plate with 1 lit capacity Top made of SS with Teflon Bed 1 Make- HB 14. Centrifuge ( 4 holders) 1 Make- HB 15. Single pan Balance ( pocket type) max: 0-500 gm, L.C: 0.1 gm 1 16. High pressure vacuum Pump 1 17. BOD Incubators inner complete S.S outer MS powder coated.( heavy) 2 Make – HB 18. Heating Mental 1 lit capacity 1 Make- HB 19. S.S distillation Unit 1 Address: 140, Tribhuvan Estate opp Road No 11 Kathwada GIDC Contact: +91 9726943972 Email: [email protected] Mfg & Exporters: All types of Turnkey Projects, , Beverages, Mineral Water plant & OEM Spares List Of Chemicals Sr. -

Signature Redacted Department of Metallurgy Gau, R E Da Signature of Professor in Charge of Research
THERMODYNAMIC PROPERTIES OF THE GROUP VIa SULFIDES: CrS, Mo2 S3 AND WS2 by JOHN PATRICK HAGER B. S., Montana School of Mines (1958) M. S. , School of Mines and Metallurgy University of Missouri (1960) Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF SCIENCE at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY August, 1969 Signature of Author Signature redacted Department of Metallurgy gau, r e da Signature of Professor in Charge of Research Signature of Chairman of Departmental Committee Graduate Research nchives Signature redacted INST. 7 DEC 2 1969 4IBRARIES -~ U ii THERMODYNAMIC PROPERTIES OF THE GROUP VIa SULFIDES: CrS, Mo 2S 3 and WS 2 by John Patrick Hager Submitted to the Department of Metallurgy on August 18, 1969, in partial fulfillment of the requirements for the degree of Doctor of Science. ABSTRACT The thermodynamic properties of the metal-saturated phase of the Group VIa sulfides have been determined through a study of the effect of gas composition on the sulfidizing action of (H2S g) + H 2 (g) ) mixtures passed over heated metal samples. The following equations for the standard free energy of formation of CrS (c) ' Mo2S3(c) and WS2(c) were obtained: AFCc) ( 250) = -48,190 ( 760) + 13.27( 0.52)T; cal/l/2 g-mole S2(g) (1375-1570 0 K) AF 0 ( 220) = -41,730( 890) + 17.39( 0.59)1R; cal/l/2 g-mole S2(g) 102S3 (c)2(g (1365-1610 0 K) AF ( 220) = -40,110( 920) + 18.64( 0.63)T; cal/l/2 g-mole S WS 2 (c) 2(g) (1370-15650K) An additional study of the thermodynamic properties of Cu2S ) pro- vided a means of evaluating the experimental technique.