Does Ethylene Treatment Mimic the Effects of Pollination on Floral Lifespan and Attractiveness?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

1 the Global Flower Bulb Industry
1 The Global Flower Bulb Industry: Production, Utilization, Research Maarten Benschop Hobaho Testcentrum Hillegom, The Netherlands Rina Kamenetsky Department of Ornamental Horticulture Agricultural Research Organization The Volcani Center Bet Dagan 50250, Israel Marcel Le Nard Institut National de la Recherche Agronomique 29260 Ploudaniel, France Hiroshi Okubo Laboratory of Horticultural Science Kyushu University 6-10-1 Hakozaki, Higashi-ku Fukuoka 812-8581, Japan August De Hertogh Department of Horticultural Science North Carolina State University Raleigh, NC 29565-7609, USA COPYRIGHTED MATERIAL I. INTRODUCTION II. HISTORICAL PERSPECTIVES III. GLOBALIZATION OF THE WORLD FLOWER BULB INDUSTRY A. Utilization and Development of Expanded Markets Horticultural Reviews, Volume 36 Edited by Jules Janick Copyright Ó 2010 Wiley-Blackwell. 1 2 M. BENSCHOP, R. KAMENETSKY, M. LE NARD, H. OKUBO, AND A. DE HERTOGH B. Introduction of New Crops C. International Conventions IV. MAJOR AREAS OF RESEARCH A. Plant Breeding and Genetics 1. Breeders’ Right and Variety Registration 2. Hortus Bulborum: A Germplasm Repository 3. Gladiolus 4. Hyacinthus 5. Iris (Bulbous) 6. Lilium 7. Narcissus 8. Tulipa 9. Other Genera B. Physiology 1. Bulb Production 2. Bulb Forcing and the Flowering Process 3. Morpho- and Physiological Aspects of Florogenesis 4. Molecular Aspects of Florogenesis C. Pests, Physiological Disorders, and Plant Growth Regulators 1. General Aspects for Best Management Practices 2. Diseases of Ornamental Geophytes 3. Insects of Ornamental Geophytes 4. Physiological Disorders of Ornamental Geophytes 5. Exogenous Plant Growth Regulators (PGR) D. Other Research Areas 1. Specialized Facilities and Equipment for Flower Bulbs52 2. Transportation of Flower Bulbs 3. Forcing and Greenhouse Technology V. MAJOR FLOWER BULB ORGANIZATIONS A. -

Dyuhei Sato Division of Genetics, Bot. Inst. Faculty of Science, Tokyo
ANALYSIS OF THE KARYOTYPES IN YUCCA, A GA VE AND THE RELATED GENERA WITH SPECIAL REFERENCE TO THE PHYLOGENETIC SIGNIFICANCEI~ Dyuhei SATo Divisionof Genetics, Bot. Inst. Faculty of Science, Tokyo Imperial University McKelvey and Sax (2933) have called attention to the existence of taxonomic and cytological similarities of the genera Yucca, Hesperoyucca, Gleistvucca,Hesperoaloe and Samuela of the Liliaceae with the genera Agave and Fourcroya which belong to a related family, Amaryllidaceae. Wh.itaker (1934) also has reported that Polianhes and Fourcroya have exactly the same chromosome constitution as the Yucca-Abave karyotype (5 long and 25 short chromosomes) (Figs. 1, 2). These observations when considered in respect to taxonomic resemblances, seem to indicate that the genera mentioned above are more closely related than it is shown by their classifica- tion into distinct families. Whitaker also has remarked that Dasylirion (2n=38) and ATolina(2n=36) in Yucceae and Doryanthes (2n=36) in Agavoideae are of different karyotypes from the Yucca-Agave type. In the present work an analysis of the karyotypes in Liliaceous plants has been attempted and several karyotypes have been found in Scilloideae. Eucornis and Carassia have been selected with the purpose of discovering a possible connecting link between these genera and the Yucca-Agave group. In the present paper an analysis of the karyotypes of the following species is given. LILIACEAE Scilloideae 211 Fig. Euconis undulata 60=8L+8M+44S (4b)2) 3 Euconsispallidi ora 60=8L+8M+44S (4b) 4 Eucomispunctata 60=8L±8M+44S (4b) 5 Camassiaescrema 30=6L+24S (2b) 6 Yucceae Yuccafilamentosa 30 60=1OL+50S (2b) 1, 7 Yuccarecurvifolia 30 60=1OL+50S (2b) 2, 8 Yuccaaloifolia 60=1OL+50S (2b) 9 „ var. -

11 Emes RESPONSE of Ornithogalum Saundersiae Bak. TO
ISSN 1644-0692 www.acta.media.pl Acta Sci. Pol. Hortorum Cultus, 15(1) 2016, 123-134 RESPONSE OF Ornithogalum saundersiae BAK. TO SALINITY STRESS Piotr Salachna, Agnieszka Zawadzi ńska, Cezary Podsiadło 1 West Pomeranian University of Technology in Szczecin Abstract. Most of the studies on the effects of salinity stress are conducted on ornamental bedding plants and perennials but little is known on flower bulbs response to this stress factor. Ornithogalum saundersiae is an attractive bulbous plant recommended for grow- ing in pots, gardens and green areas. The study conducted in the years 2013–2014 investi- gated the effects of NaCl on the growth, flowering, photosynthetic activity, pigment con- tent, and macro- and micronutrient content in the leaves of O. saundersiae . The plants were grown in pots in a plastic tunnel. NaCl was applied once a week for six weeks at concentration of 100 mM or 200 mM. The salt treatment did not cause chlorosis and did not affect flowering rate and number of inflorescences. The plants exposed to salinity stress had lower fresh weight of leaves, inflorescences and bulbs and their flowering be- gan later than in the control plants. Photosynthesis and transpiration intensity decreased as NaCl concentration increased. The content of chlorophyll and carotenoids in NaCl treated plants was significantly higher than in the control plants. Salinity stress increased the leaf content of nitrogen, potassium, sodium and chlorine and reduced the concentration of cal- cium, zinc and iron. Key words: Giant Chincherinchee, NaCl, gas exchange, mineral content, photosynthetic pig- ments INTRODUCTION The issue of salinity stress in the cultivation of ornamental plants is receiving global attention [Niu and Cabrera 2010, Cassaniti et al. -

Rutgers Home Gardeners School: the Beauty of Bulbs
The Beauty of Bulbs Bruce Crawford March 17, 2018 Director, Rutgers Gardens Rutgersgardens.rutgers.edu In general, ‘bulbs’, or more properly, geophytes are easy plants to grow, requiring full sun, good drainage and moderately fertile soils. Geophytes are defined as any non-woody plant with an underground storage organ. These storage organs contain carbohydrates, nutrients and water and allow the plant to endure extended periods of time that are not suitable for plant growth. Types of Geophytes include: Bulb – Swollen leaves or leaf stalks, attached at the bottom to a modified stem called a basal plant. The outer layers are modified leaves called scales. Scales contain necessary foods to sustain the bulb during dormancy and early growth. The outermost scales become dry and form a papery covering or tunic. At the center are developed, albeit embryonic flowers, leaves and stem(s). Roots develop from the basal plate. Examples are Tulipia (Tulip), Narcissus (Daffodil), and Allium (Flowering Onion). Corm – A swollen stem that is modified for food storage. Eyes or growing points develop on top of the corm. Roots develop from a basal plate on the bottom of the corm, similar to bulbs. The dried bases of the leaves from an outer layer, also called the tunic. Examples include Crocus and Erythronium (Dog Tooth Violet). Tuber – Also a modified stem, but it lacks a basal plate and a tunic. Roots, shoots and leaves grow from eyes. Examples are Cyclamen, Eranthis (Winter Aconite) and Anemone (Wind Flower). Tuberous Roots – These enlarged storage elements resemble tubers but are swollen roots, not stems. During active growth, they produce a fibrous root system for water and nutrient absorption. -
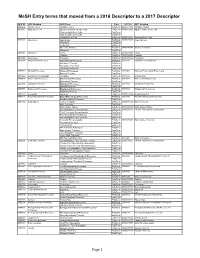
Mesh Entry Terms That Moved from a 2016 Descriptor to a 2017 Descriptor
MeSH Entry terms that moved from a 2016 Descriptor to a 2017 Descriptor 2016 UI 2016 Heading 2016 Term Type 2017 UI 2017 Heading D000204 Activity Cycles Ultradian Cycles EntryTerm D000071218 Ultradian Rhythm D053687 Adult Stem Cells Spermatogonial Progenitor Cells EntryTerm D000072956 Adult Germline Stem Cells Germline Adult Stem Cells EntryTerm Adult Germline Stem Cells EntryTerm Oogonial Stem Cells EntryTerm D000072977 Oogonial Stem Cells D027881 Agavaceae Agavaceae PrefTerm D000070357 Asparagaceae Polianthes EntryTerm Hesperaloe EntryTerm Flax, New Zealand EntryTerm D000070380 Xanthorrhoeaceae Phormium EntryTerm D000383 Agriculture Farms EntryTerm D000072480 Farms Gardens EntryTerm D000072506 Gardens D019659 Asteraceae Psacalium EntryTerm D000070216 Psacalium D055588 Astronomical Processes Astronomical Processes PrefTerm D055580 Astronomical Phenomena Astronomic Process EntryTerm Astronomic Processes EntryTerm Astronomical Process EntryTerm D055683 Bacterial Processes Bacterial Processes PrefTerm D018407 Bacterial Physiological Phenomena Bacterial Process EntryTerm D001626 Bethanechol Compounds Urecholine EntryTerm D018723 Bethanechol D055438 Biochemical Processes Biochemical Processes PrefTerm D001669 Biochemical Phenomena Biochemical Process EntryTerm D055694 Biological Processes Biological Processes PrefTerm D001686 Biological Phenomena Biological Process EntryTerm D055597 Biophysical Processes Biophysical Processes PrefTerm D055592 Biophysical Phenomena Biophysical Process EntryTerm D001727 Bisexuality Bisexuals EntryTerm D000072339 -

Una Nueva Especie De Tanqua Karoo (Sudáfrica), Africa), with Notes on E
2311_Ethesia_Mnez.Azo.af_Anales 69(2).qxd 14/12/2012 12:38 Página 201 Anales del Jardín Botánico de Madrid 69(2): 201-208, julio-diciembre 2012. ISSN: 0211-1322. doi: 10.3989/ajbm. 2311 Ethesia tanquana (Ornithogaloideae, Hyacinthaceae), a new species from the Tanqua Karoo (South Africa), with notes on E. haalenbergensis Mario Martínez-Azorín* & Manuel B. Crespo CIBIO (Instituto de la Biodiversidad), Universidad de Alicante, Apartado 99, E-03080 Alicante, Spain; [email protected] Abstract Resumen Martínez-Azorín, M. & Crespo, M.B. 2012. Ethesia tanquana (Ornithoga- Martínez-Azorín, M. & Crespo, M.B. 2012. Ethesia tanquana (Ornithoga- loideae, Hyacinthaceae), a new species from the Tanqua Karoo (South loideae, Hyacinthaceae), una nueva especie de Tanqua Karoo (Sudáfrica), Africa), with notes on E. haalenbergensis. Anales Jard. Bot. Madrid 69(2): con notas sobre E. haalenbergensis. Anales Jard. Bot. Madrid 69(2): 201- 201-208. 208 (en inglés). As a part of a taxonomic revision of Ethesia Raf., a new species, E. tanqua- En el marco de la revisión taxonómica de Ethesia Raf., se describe una nue- na Mart.-Azorín & M.B.Crespo, is described from the Tanqua Karoo in va especie, E. tanquana Mart.-Azorín & M.B.Crespo, del Tanqua Karoo en South Africa. This new species is at first sight similar to E. haalenbergensis Sudáfrica. Esta nueva especie se asemeja a primera vista a E. haalenber- (U.Müll.-Doblies & D.Müll.-Doblies) Mart.-Azorín, M.B.Crespo & Juan and gensis (U.Müll.-Doblies & D.Müll.-Doblies) Mart.-Azorín, M.B.Crespo & also E. xanthochlora (Baker) Mart.-Azorín, M.B.Crespo & Juan, but it dif- Juan y E. -

T H a I S Z I a Flower Morphology and Vascular Anatomy in Some
Thaiszia - J. Bot., Košice, 28 (2): 125-143, 2018 http://www.bz.upjs.sk/thaiszia T H A I S Z I A JOURNAL OF BOTANY Flower morphology and vascular anatomy in some representatives of Urgineoideae (Hyacinthaceae) OLGA DYKA1 1Ivan Franko National University of Lviv, 4, Hrushevsky str., 79005, Lviv, Ukraine; [email protected] Dyka O. (2018): Flower morphology and vascular anatomy in some representatives of Urgineoideae (Hyacinthaceae). – Thaiszia – J. Bot. 28 (2): 125-143. – ISSN 1210-0420. Abstract: The flower morphology and vascular anatomy in Bowiea volubilis, Geschollia anomala and Fusifilum physodes have been studied. It was shown that the perigonium and androecium in the studied species are very similar. Each of tepals, and each stamen is supplied by a single vascular bundle. Some significant differences are recognized in the inner structure of the ovary, vertical zonality of septal nectary, as well as gynoecium venation in the studied species. Accordingly to W. Leinfellner concept of the gynoecium vertical zonality, it was established that the gynoecium of B. volubilis consists of hemisynascidiate, hemisymplicate and asymplicate zones, and the gynoecium of G. anomala and F. physodes consists of synascidiate, symplicate, hemisymplicate and asymplicate zones. The septal nectary of all studied species have a nectary cavity and nectary split which opens to the exterior. In the septal nectary we detected three vertical zones: the zone of the distinct nectary at the symplicate zone in F. physodes; the zone of the common nectary at the hemisynascidiate, and hemisymplicate zones in B. volubilis, and at the hemisymplicate zone in G. anomala and F. physodes; the zone of the external nectary (nectary splits) in all studied species at the asymplicate zone in the ovary roof. -

Pacific Bulb Society Bulb and Seed Exchange (BX) 201-300 Details for Items Listed Here Have Been Truncated Due to Space Contraints
Pacific Bulb Society Bulb and ExchangeSeed (BX) 201-300 6. 5. 4. 3. 2. 1. >FromPBS: BX 201 itemsfor Winter= 204. itemsfor = 269,Spring total itemsSummer for = 695, total items for Autumn = 1002, total itemper =21.7,BX itemsaverage per month = 65.7, BX’saverage month = 3,per total Thefollowing are statistical analyses of BX201-300, 2009-2011. itemTotal =2170, average andsearch for item the in appropriate the BX. descriptionsof each item, visit PBS the archives ( Detail >FromMary Ittner:Sue (BULBS) 9. 8. 7. 6. 5. 4. 3. 2. 1. >FromPBS: (SEEDS) BX 202 13. 12. 11. 10. 9. 8. 7. Eucomis zambesiaca Dieramaigneum Geissorhizaovata Babianamucronata Brunsvigiajosephinae Boophanehaemanthoides Albucasetosa Moraeahuttoniae Drimiauniflora Aristeawoodii Dieramadracomontanum Hypoxishemerocallidea Agapanthus inapertus Ornithogalumthyrsoides Kniphofiasarmentosa Lachenaliaaurioliae Ixiaorientalis Eriospermumconfusum Items 10 20 30 40 50 60 Tulbaghiaalliacea Polyxenaensilfolia ssp. maughamii Moraealugubris Lachenaliaperryae 0 March 2009 s items for listed herehave been truncated due spaceto contraints. For moredetailed May 2009 (April 2009) 17, (March 30, 2009) June 2009 July 2009 July 2009 July 2009 , short , form August 2009 August 2009 September 2009 September 2009 October 2009 October 2009 November 2009 November 2009 December 2009 February 2010 March 2010 April 2010 May 2010 May 2010 June 2010 PBS BX 200-300 BX PBS July 2010 July 2010 August 2010 Date August 2010 August 2010 http://www.pacificbulbsociety.org/list.php 7. filipponei 6. 5. 4. >FromLynn Makela: (BULBS) 3.Bulbs of >FromMary Ittner: Sue 2.Seed of >FromDell Sherk: humilis 1.Small bulbs of >FromJim Shields: BX 203 15. 14. 13. SEEDS: montanus 12.Bulblets of 11. 10. September 2010 Ipheionsessile Ipheionsellowianum Habranthusbrachyandrus Achimenesgrandiflora October 2010 Massoniajasminiflora Hesperoxiphionperuvianum Haemanthusalbiflos Oxalis Nerinemasoniorum October 2010 November 2010 November 2010 ) (May 2009) 5, December 2010 sp. -

Exposing Seeds of Galtonia Candicans to Ethyl
HORTSCIENCE 55(5):621–624. 2020. https://doi.org/10.21273/HORTSCI14775-19 tive to varying concentrations of EMS treatment, as well as changes in seed dor- Galtonia candicans mancy (Greer and Rinehart, 2009; Hoskins Exposing Seeds of to and Contreras, 2019; Talebi et al., 2012). Weigle and Butler (1983) reported heredi- Ethyl Methanesulfonate Reduced tary dwarfing effects after EMS treatment of Impatiens platypeta seeds and in Capsicum Inflorescence Height, Lodging, annuum, Jabeen and Mirza (2004) demonstrated that EMS was effective in inducing dwarf mutations and sterility. These results, if ob- and Fertility served in cape hyacinth, would result in im- Ryan N. Contreras proved cultivars with potential as containerized Department of Horticulture, Oregon State University, 4017 Agricultural and summer-flowering perennials for retail sales, reduced lodging to improve landscape perfor- Life Sciences, Corvallis, OR 97331 mance, and reduced fertility to prevent escape Kim Shearer from cultivation. The objective of this study was to evaluate the effects of increasing concentra- The Morton Arboretum, 4100 Illinois Route 53, Lisle, IL 60532 tions of EMS on G. candicans for these traits. Additional index words. cape hyacinth, chemical mutagens, Hyacinthaceae, mutagenesis, sterility Materials and Methods Abstract. Cape hyacinth (Galtonia candicans) is a geophytic herbaceous perennial from Mutagenesis and germination. In 2011, South Africa. It produces large inflorescences of pendulous white flowers during mid to seed from landscape plants of G. candicans at late summer, followed by capsules filled with copious amounts of seed. The species has Oregon State University North Willamette potential as a low-water-use landscape plant, but lodging and excessive seed production, Research and Extension Center (Aurora, OR) which pose a risk of escape or invasion, are issues that should be addressed before were collected. -

Hyacinthaceae | Plantz Africa About:Reader?Url=
Hyacinthaceae | Plantz Africa about:reader?url=http://pza.sanbi.org/hyacinthaceae pza.sanbi.org Hyacinthaceae | Plantz Africa Hyacinthaceae Family: Hyacinthaceae Common names: hyacinth family Introduction This is a family of 700-900 species of deciduous, or rarely evergreen, bulbous plants, several with brightly coloured flowers, that is well represented in South Africa. Description Description The plants are deciduous or rarely evergreen perennials with a bulb, which can sometimes be quite large. The leaves are usually lance-shaped and soft-textured, with rather slimy sap. The leaves are mostly held upright but in several species from the South African winter rainfall area they lie flat against the ground. Mostly the leaves are smooth and unmarked but in most species of Ledebouria (also some Lachenalia and Eucomis from South Africa) the leaves are attractively spotted or streaked with purple or dark green. Occasionally the leaves may have their upper surface covered with warts or pustules, or coarse hairs, especially in species of Lachenalia and Massonia . In some species the leaves are narrow and needle-like or cylindrical. The flower stalk is leafless and the flowers are always arranged in racemes. Sometimes these may be very short so that the flowers are crowded into a head-like cluster. In the genera Bowiea and Igidiae the raceme is highly branched and sprawls through the surrounding vegetation. Each flower arises in the axil of a bract, which may be large and leaf-like or minute and vestigial. In the subfamily Urgineoideae the lower bracts have a flattened spur at their base. In some genera a second smaller bract arises on the base of the flower stalk, or pedicel, as well. -

Flowering Plants of Africa
Flowering Plants of Africa A magazine containing colour plates with descriptions of flowering plants of Africa and neighbouring islands Edited by G. Germishuizen with assistance of E. du Plessis and G.S. Condy Volume 60 Pretoria 2007 Editorial Board B.J. Huntley formerly South African National Biodiversity Institute, Cape Town, RSA G.Ll. Lucas Royal Botanic Gardens, Kew, UK B. Mathew Royal Botanic Gardens, Kew, UK Referees and other co-workers on this volume C. Archer, South African National Biodiversity Institute, Pretoria, RSA H. Beentje, Royal Botanic Gardens, Kew, UK C.L. Bredenkamp, South African National Biodiversity Institute, Pretoria, RSA P.V. Bruyns, Bolus Herbarium, Department of Botany, University of Cape Town, RSA P. Chesselet, Muséum National d’Histoire Naturelle, Paris, France C. Craib, Bryanston, RSA A.P. Dold, Botany Department, Rhodes University, Grahamstown, RSA G.D. Duncan, South African National Biodiversity Institute, Cape Town, RSA V.A. Funk, Department of Botany, Smithsonian Institution, Washington DC, USA P. Goldblatt, Missouri Botanical Garden, St Louis, Missouri, USA S. Hammer, Sphaeroid Institute, Vista USA C. Klak, Department of Botany, University of Cape Town, RSA M. Koekemoer, South African National Biodiversity Institute, Pretoria, RSA O.A. Leistner, c/o South African National Biodiversity Institute, Pretoria, RSA S. Liede-Schumann, Department of Plant Systematics, University of Bayreuth, Germany J.C. Manning, South African National Biodiversity Institute, Cape Town, RSA D.C.H. Plowes, Mutare, Zimbabwe E. Retief, South African National Biodiversity Institute, Pretoria, RSA S.J. Siebert, Department of Botany, University of Zululand, KwaDlangezwa, RSA D.A. Snijman, South African National Biodiversity Institute, Cape Town, RSA C.D.