Micropropagation of Members of the Hyacinthaceae with Medicinal and Ornamental Potential- a Review
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

SOUTHERN CALIFORNIA HORTICULTURAL SOCIETY Where Passionate Gardeners Meet to Share Knowledge and Learn from Each Other
SOUTHERN CALIFORNIA HORTICULTURAL SOCIETY Where passionate gardeners meet to share knowledge and learn from each other. socalhort.org June 2013 Newsletter OUR NEXT MEETING PLANT FORUM NEXT SHARING SECRETS Bring one or more plants, QUESTION Thursday, June 13 flowers, seeds or fruits for IN THIS ISSUE Inspired by this month’s 7:30 pm display and discussion at the program, the Sharing Secrets May Meeting Recap Friendship Auditorium Plant Forum. We will soon have question for June is: by Steven Gerischer ............... 2 3201 Riverside Drive an improved, downloadable Sharing Secrets ......................... 2 Los Angeles CA 90027 PDF version of the plant "Do you preserve any of the information card. Anyone produce you grow, and Coffee in the Garden................2 We meet the second Thursday bringing in material for the how?” Upcoming Field Trips & Coffee In of each month at 7:30 pm Plant Forum table should ______________________________ The Garden ............................... 2 remember to pick up an You can answer on the cards March 2013 Green Sheet by This meeting is free to SCHS exhibitor’s ticket for the Plant we’ll supply at our June 13 James E. Henrich............3, 4 & 5 members and is $5 for non- Raffle, on nights when a raffle meeting, on our MemberLodge members without a guest pass. is conducted. These plants are website or e-mail your Horticultural Happenings also included in our response to by Bettina Gatti ........................6 newsletter’s Green Sheet. [email protected] by Friday, Upcoming 2013 SCHS June 14. Programs ................................... 7 The June Meeting In the 21st century we take food PLANT RAFFLE RETURNS! preservation for granted. -

Ciencias Ambientales Y Recursos Naturales
DEPARTAMENTO DE: CIENCIAS AMBIENTALES Y RECURSOS NATURALES Director: ROJO VELASCO, SANTOS AÑO DE LA MEMORIA: 2014 PERSONAL INVESTIGADOR 1. ALONSO VARGAS, MARIA ANGELES 2. BORDERA SANJUAN, SANTIAGO 3. CASAS MARTINEZ, JOSE LUIS 4. CRESPO VILLALBA, MANUEL BENITO 5. DE LA TORRE GARCIA, ANTONIO 6. GALANTE PATIÑO, EDUARDO 7. GUERRERO MARTINEZ, JUAN RAMON 8. JUAN GALLARDO, ANA ISABEL 9. MARCOS GARCIA, MARIA DE LOS ANGELES 10. MARTINEZ AZORIN, MARIO 11. MARTINEZ SANCHEZ, ANA ISABEL 12. MICO BALAGUER, ESTEFANIA 13. PEREZ BAÑON, MARIA CELESTE 14. PEREZ BOTELLA, JOAN 15. PIQUERAS CASTILLO, ABEL 16. RIOS RUIZ, SEGUNDO 17. ROJO VELASCO, SANTOS 18. SERNA GUIRAO, MARIA DOLORES 19. SOLANAS FERRANDIZ, JOSE LUIS 20. TERRONES CONTRERAS, ALEJANDRO 21. URIOS MOLINER, VICENTE 22. VERDU FARACO, JOSE RAMON LÍNEAS DE INVESTIGACIÓN 1. Biología de Himenópteros Parasitoides. 2. Biología floral y reproductiva. 3. Biología y ecología de hongos entomófagos y nematófagos. 4. Biología y Ecología de insectos descomponedores. 5. Biota marina (taxonomía, biología, ecología, biogeografía) 6. Comunidades bentónicas. 7. Conservación de germoplasma vegetal. 8. Control biológico de patógenos vegetales y plagas por hongos. 9. Ecofisiología vegetal: respuestas y adaptaciones de las plantas al estrés. Página 1 Fecha de actualización 19/06/2018 Universidad de Alicante CIENCIAS AMBIENTALES Y RECURSOS NATURALES 2014 10. Estrategia de conservación de flora endémica, rara o amenazada. 11. Estudio biodiversidad y conservación animal. 12. Estudio ecosistemas mediterráneos. 13. Estudios de la fisiológia de plantas durante la post–recolección de frutos, flores y hortalizas. 14. Fitogeografía y Geobotánica. 15. Fitopatología. 16. Fitosociología. 17. Flora y vegetación del sureste de España. 18. Hongos fitopatogenos de palmáceas. -

1 the Global Flower Bulb Industry
1 The Global Flower Bulb Industry: Production, Utilization, Research Maarten Benschop Hobaho Testcentrum Hillegom, The Netherlands Rina Kamenetsky Department of Ornamental Horticulture Agricultural Research Organization The Volcani Center Bet Dagan 50250, Israel Marcel Le Nard Institut National de la Recherche Agronomique 29260 Ploudaniel, France Hiroshi Okubo Laboratory of Horticultural Science Kyushu University 6-10-1 Hakozaki, Higashi-ku Fukuoka 812-8581, Japan August De Hertogh Department of Horticultural Science North Carolina State University Raleigh, NC 29565-7609, USA COPYRIGHTED MATERIAL I. INTRODUCTION II. HISTORICAL PERSPECTIVES III. GLOBALIZATION OF THE WORLD FLOWER BULB INDUSTRY A. Utilization and Development of Expanded Markets Horticultural Reviews, Volume 36 Edited by Jules Janick Copyright Ó 2010 Wiley-Blackwell. 1 2 M. BENSCHOP, R. KAMENETSKY, M. LE NARD, H. OKUBO, AND A. DE HERTOGH B. Introduction of New Crops C. International Conventions IV. MAJOR AREAS OF RESEARCH A. Plant Breeding and Genetics 1. Breeders’ Right and Variety Registration 2. Hortus Bulborum: A Germplasm Repository 3. Gladiolus 4. Hyacinthus 5. Iris (Bulbous) 6. Lilium 7. Narcissus 8. Tulipa 9. Other Genera B. Physiology 1. Bulb Production 2. Bulb Forcing and the Flowering Process 3. Morpho- and Physiological Aspects of Florogenesis 4. Molecular Aspects of Florogenesis C. Pests, Physiological Disorders, and Plant Growth Regulators 1. General Aspects for Best Management Practices 2. Diseases of Ornamental Geophytes 3. Insects of Ornamental Geophytes 4. Physiological Disorders of Ornamental Geophytes 5. Exogenous Plant Growth Regulators (PGR) D. Other Research Areas 1. Specialized Facilities and Equipment for Flower Bulbs52 2. Transportation of Flower Bulbs 3. Forcing and Greenhouse Technology V. MAJOR FLOWER BULB ORGANIZATIONS A. -

Dyuhei Sato Division of Genetics, Bot. Inst. Faculty of Science, Tokyo
ANALYSIS OF THE KARYOTYPES IN YUCCA, A GA VE AND THE RELATED GENERA WITH SPECIAL REFERENCE TO THE PHYLOGENETIC SIGNIFICANCEI~ Dyuhei SATo Divisionof Genetics, Bot. Inst. Faculty of Science, Tokyo Imperial University McKelvey and Sax (2933) have called attention to the existence of taxonomic and cytological similarities of the genera Yucca, Hesperoyucca, Gleistvucca,Hesperoaloe and Samuela of the Liliaceae with the genera Agave and Fourcroya which belong to a related family, Amaryllidaceae. Wh.itaker (1934) also has reported that Polianhes and Fourcroya have exactly the same chromosome constitution as the Yucca-Abave karyotype (5 long and 25 short chromosomes) (Figs. 1, 2). These observations when considered in respect to taxonomic resemblances, seem to indicate that the genera mentioned above are more closely related than it is shown by their classifica- tion into distinct families. Whitaker also has remarked that Dasylirion (2n=38) and ATolina(2n=36) in Yucceae and Doryanthes (2n=36) in Agavoideae are of different karyotypes from the Yucca-Agave type. In the present work an analysis of the karyotypes in Liliaceous plants has been attempted and several karyotypes have been found in Scilloideae. Eucornis and Carassia have been selected with the purpose of discovering a possible connecting link between these genera and the Yucca-Agave group. In the present paper an analysis of the karyotypes of the following species is given. LILIACEAE Scilloideae 211 Fig. Euconis undulata 60=8L+8M+44S (4b)2) 3 Euconsispallidi ora 60=8L+8M+44S (4b) 4 Eucomispunctata 60=8L±8M+44S (4b) 5 Camassiaescrema 30=6L+24S (2b) 6 Yucceae Yuccafilamentosa 30 60=1OL+50S (2b) 1, 7 Yuccarecurvifolia 30 60=1OL+50S (2b) 2, 8 Yuccaaloifolia 60=1OL+50S (2b) 9 „ var. -

Insights from Microsporogenesis in Asparagales
EVOLUTION & DEVELOPMENT 9:5, 460–471 (2007) Constraints and selection: insights from microsporogenesis in Asparagales Laurent Penet,a,1,Ã Michel Laurin,b Pierre-Henri Gouyon,a,c and Sophie Nadota aLaboratoire Ecologie, Syste´matique et Evolution, Batiment 360, Universite´ Paris-Sud, 91405 Orsay Ce´dex, France bUMR CNRS 7179, Universite´ Paris 6FPierre & Marie Curie, 2 place Jussieu, Case 7077, 75005 Paris, France cMuse´um National d’Histoire Naturelle, De´partement de Syste´matique et Evolution Botanique, 12 rue Buffon, 75005 Paris CP 39, France ÃAuthor for correspondence (email: [email protected]) 1Current address: Department of Biological Sciences, University of Pittsburgh, 4249 Fifth & Ruskin, Pittsburgh, PA 15260, USA. SUMMARY Developmental constraints have been proposed different characteristics of microsporogenesis, only cell to interfere with natural selection in limiting the available wall formation appeared as constrained. We show that set of potential adaptations. Whereas this concept has constraints may also result from biases in the correlated long been debated on theoretical grounds, it has been occurrence of developmental steps (e.g., lack of successive investigated empirically only in a few studies. In this article, cytokinesis when wall formation is centripetal). We document we evaluate the importance of developmental constraints such biases and their potential outcomes, notably the during microsporogenesis (male meiosis in plants), with an establishment of intermediate stages, which allow emphasis on phylogenetic patterns in Asparagales. Different development to bypass such constraints. These insights are developmental constraints were tested by character discussed with regard to potential selection on pollen reshuffling or by simulated distributions. Among the morphology. INTRODUCTION 1991) also hindered tests using the concept (Pigliucci and Kaplan 2000). -

11 Emes RESPONSE of Ornithogalum Saundersiae Bak. TO
ISSN 1644-0692 www.acta.media.pl Acta Sci. Pol. Hortorum Cultus, 15(1) 2016, 123-134 RESPONSE OF Ornithogalum saundersiae BAK. TO SALINITY STRESS Piotr Salachna, Agnieszka Zawadzi ńska, Cezary Podsiadło 1 West Pomeranian University of Technology in Szczecin Abstract. Most of the studies on the effects of salinity stress are conducted on ornamental bedding plants and perennials but little is known on flower bulbs response to this stress factor. Ornithogalum saundersiae is an attractive bulbous plant recommended for grow- ing in pots, gardens and green areas. The study conducted in the years 2013–2014 investi- gated the effects of NaCl on the growth, flowering, photosynthetic activity, pigment con- tent, and macro- and micronutrient content in the leaves of O. saundersiae . The plants were grown in pots in a plastic tunnel. NaCl was applied once a week for six weeks at concentration of 100 mM or 200 mM. The salt treatment did not cause chlorosis and did not affect flowering rate and number of inflorescences. The plants exposed to salinity stress had lower fresh weight of leaves, inflorescences and bulbs and their flowering be- gan later than in the control plants. Photosynthesis and transpiration intensity decreased as NaCl concentration increased. The content of chlorophyll and carotenoids in NaCl treated plants was significantly higher than in the control plants. Salinity stress increased the leaf content of nitrogen, potassium, sodium and chlorine and reduced the concentration of cal- cium, zinc and iron. Key words: Giant Chincherinchee, NaCl, gas exchange, mineral content, photosynthetic pig- ments INTRODUCTION The issue of salinity stress in the cultivation of ornamental plants is receiving global attention [Niu and Cabrera 2010, Cassaniti et al. -

Rutgers Home Gardeners School: the Beauty of Bulbs
The Beauty of Bulbs Bruce Crawford March 17, 2018 Director, Rutgers Gardens Rutgersgardens.rutgers.edu In general, ‘bulbs’, or more properly, geophytes are easy plants to grow, requiring full sun, good drainage and moderately fertile soils. Geophytes are defined as any non-woody plant with an underground storage organ. These storage organs contain carbohydrates, nutrients and water and allow the plant to endure extended periods of time that are not suitable for plant growth. Types of Geophytes include: Bulb – Swollen leaves or leaf stalks, attached at the bottom to a modified stem called a basal plant. The outer layers are modified leaves called scales. Scales contain necessary foods to sustain the bulb during dormancy and early growth. The outermost scales become dry and form a papery covering or tunic. At the center are developed, albeit embryonic flowers, leaves and stem(s). Roots develop from the basal plate. Examples are Tulipia (Tulip), Narcissus (Daffodil), and Allium (Flowering Onion). Corm – A swollen stem that is modified for food storage. Eyes or growing points develop on top of the corm. Roots develop from a basal plate on the bottom of the corm, similar to bulbs. The dried bases of the leaves from an outer layer, also called the tunic. Examples include Crocus and Erythronium (Dog Tooth Violet). Tuber – Also a modified stem, but it lacks a basal plate and a tunic. Roots, shoots and leaves grow from eyes. Examples are Cyclamen, Eranthis (Winter Aconite) and Anemone (Wind Flower). Tuberous Roots – These enlarged storage elements resemble tubers but are swollen roots, not stems. During active growth, they produce a fibrous root system for water and nutrient absorption. -

Plethora of Plants – Collections of the Botanical Garden, Faculty Of
Nat. Croat. Vol. 24(2), 2015 361 NAT. CROAT. VOL. 24 No 2 361–397* ZAGREB December 31, 2015 professional paper / stručni članak – museal collections / muzejske zbirke DOI: 10.302/NC.2015.24.26 PLETHORA OF PLANTS – ColleCtions of the BotaniCal Garden, faCulty of ScienCe, university of ZaGreB (1): temperate Glasshouse exotiCs – HISTORIC OVERVIEW Sanja Kovačić Botanical Garden, department of Biology, faculty of science, university of Zagreb, marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Kovačić, S.: Plethora of plants – collections of the Botanical garden, Faculty of Science, Univer- sity of Zagreb (1): Temperate glasshouse exotics – historic overview. Nat. Croat., Vol. 24, No. 2, 361–397*, 2015, Zagreb due to the forthcoming obligation to thoroughly catalogue and officially register all living and non-living collections in the european union, an inventory revision of the plant collections in Zagreb Botanical Garden of the faculty of science (university of Zagreb, Croatia) has been initiated. the plant lists of the temperate (warm) greenhouse collections since the construction of the first, exhibition Glasshouse (1891), until today (2015) have been studied. synonymy, nomenclature and origin of plant material have been sorted. lists of species grown (or that presumably lived) in the warm greenhouse conditions during the last 120 years have been constructed to show that throughout that period at least 1000 plant taxa from 380 genera and 90 families inhabited the temperate collections of the Garden. today, that collection holds 320 exotic taxa from 146 genera and 56 families. Key words: Zagreb Botanical Garden, warm greenhouse conditions, historic plant collections, tem- perate glasshouse collection Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno-matematičkog fakulteta Sve- učilišta u Zagrebu (1): Uresnice toplog staklenika – povijesni pregled. -
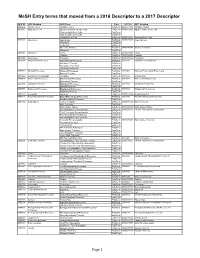
Mesh Entry Terms That Moved from a 2016 Descriptor to a 2017 Descriptor
MeSH Entry terms that moved from a 2016 Descriptor to a 2017 Descriptor 2016 UI 2016 Heading 2016 Term Type 2017 UI 2017 Heading D000204 Activity Cycles Ultradian Cycles EntryTerm D000071218 Ultradian Rhythm D053687 Adult Stem Cells Spermatogonial Progenitor Cells EntryTerm D000072956 Adult Germline Stem Cells Germline Adult Stem Cells EntryTerm Adult Germline Stem Cells EntryTerm Oogonial Stem Cells EntryTerm D000072977 Oogonial Stem Cells D027881 Agavaceae Agavaceae PrefTerm D000070357 Asparagaceae Polianthes EntryTerm Hesperaloe EntryTerm Flax, New Zealand EntryTerm D000070380 Xanthorrhoeaceae Phormium EntryTerm D000383 Agriculture Farms EntryTerm D000072480 Farms Gardens EntryTerm D000072506 Gardens D019659 Asteraceae Psacalium EntryTerm D000070216 Psacalium D055588 Astronomical Processes Astronomical Processes PrefTerm D055580 Astronomical Phenomena Astronomic Process EntryTerm Astronomic Processes EntryTerm Astronomical Process EntryTerm D055683 Bacterial Processes Bacterial Processes PrefTerm D018407 Bacterial Physiological Phenomena Bacterial Process EntryTerm D001626 Bethanechol Compounds Urecholine EntryTerm D018723 Bethanechol D055438 Biochemical Processes Biochemical Processes PrefTerm D001669 Biochemical Phenomena Biochemical Process EntryTerm D055694 Biological Processes Biological Processes PrefTerm D001686 Biological Phenomena Biological Process EntryTerm D055597 Biophysical Processes Biophysical Processes PrefTerm D055592 Biophysical Phenomena Biophysical Process EntryTerm D001727 Bisexuality Bisexuals EntryTerm D000072339 -

SABG Newsletter No. 37 July 2018
Southern African Bulb Group www.sabg.tk SABG Newsletter no. 37 July 2018 Newsletter Editor: Richard White sabg @ rjwhite .tk Contents News.......................................................................................................................1 Dates for your diary................................................................................................1 From the Editor.......................................................................................................1 Notices and Requests..............................................................................................2 Remembering Rod and Rachel.......................................................................................................2 SABG Bulb and Seed Exchange 2018............................................................................................2 Veltheimia bracteata free to members............................................................................................3 Request for hardiness experiences.................................................................................................3 Request for information about suppliers........................................................................................4 GDPR matters................................................................................................................................4 SABG meetings......................................................................................................5 Report on the Spring 2018 SABG meeting.....................................................................................5