11 Emes RESPONSE of Ornithogalum Saundersiae Bak. TO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Eucomis Bicolor Baker) an Ornamental and Medicinal Plant
Available online at www.worldscientificnews.com WSN 110 (2018) 159-171 EISSN 2392-2192 Chitosan improves growth and bulb yield of pineapple lily (Eucomis bicolor Baker) an ornamental and medicinal plant Andżelika Byczyńska Department of Horticulture, Faculty of Environmental Management and Agriculture, West Pomeranian University of Technology, Szczecin, Poland E-mail address: [email protected] ABSTRACT The wide demand for natural biostimulants encourages the search for new, alternative sources of substances with high biological activity. Chitosan can promote plant growth and root system development, enhance photosynthetic activity, increase nutrient and metabolite content. Eucomis bicolor, commonly known as the ‘pineapple lily’, is not widely known in terms of cultivation and biological activity. The aim of the experiment was to determine the effect of chitosan on growth of Eucomis bicolor. To the best of our knowledge, this is the first study to describe the effect of chitosan on morphological features of Eucomis bicolor. The results showed that soaking Eucomis bicolor bulbs in a chitosan solution before planting has stimulated the growth, flowering and yield of bulbs. Treating the plants with chitosan at 50 mg/L had the most beneficial effect on the number of leaves per plant, the relative chlorophyll content in the leaves as well as the number of bulbs per plant. Chitosan has a multi-directional, positive effect on plant growth and can be used as a potential biostimulant. Keywords: biostimulants, Eucomis bicolor, geophytes, ornamental crops, polysaccharides ( Received 31 August 2018; Accepted 14 September 2018; Date of Publication 15 September 2018 ) World Scientific News 110 (2018) 159-171 1. -

1 the Global Flower Bulb Industry
1 The Global Flower Bulb Industry: Production, Utilization, Research Maarten Benschop Hobaho Testcentrum Hillegom, The Netherlands Rina Kamenetsky Department of Ornamental Horticulture Agricultural Research Organization The Volcani Center Bet Dagan 50250, Israel Marcel Le Nard Institut National de la Recherche Agronomique 29260 Ploudaniel, France Hiroshi Okubo Laboratory of Horticultural Science Kyushu University 6-10-1 Hakozaki, Higashi-ku Fukuoka 812-8581, Japan August De Hertogh Department of Horticultural Science North Carolina State University Raleigh, NC 29565-7609, USA COPYRIGHTED MATERIAL I. INTRODUCTION II. HISTORICAL PERSPECTIVES III. GLOBALIZATION OF THE WORLD FLOWER BULB INDUSTRY A. Utilization and Development of Expanded Markets Horticultural Reviews, Volume 36 Edited by Jules Janick Copyright Ó 2010 Wiley-Blackwell. 1 2 M. BENSCHOP, R. KAMENETSKY, M. LE NARD, H. OKUBO, AND A. DE HERTOGH B. Introduction of New Crops C. International Conventions IV. MAJOR AREAS OF RESEARCH A. Plant Breeding and Genetics 1. Breeders’ Right and Variety Registration 2. Hortus Bulborum: A Germplasm Repository 3. Gladiolus 4. Hyacinthus 5. Iris (Bulbous) 6. Lilium 7. Narcissus 8. Tulipa 9. Other Genera B. Physiology 1. Bulb Production 2. Bulb Forcing and the Flowering Process 3. Morpho- and Physiological Aspects of Florogenesis 4. Molecular Aspects of Florogenesis C. Pests, Physiological Disorders, and Plant Growth Regulators 1. General Aspects for Best Management Practices 2. Diseases of Ornamental Geophytes 3. Insects of Ornamental Geophytes 4. Physiological Disorders of Ornamental Geophytes 5. Exogenous Plant Growth Regulators (PGR) D. Other Research Areas 1. Specialized Facilities and Equipment for Flower Bulbs52 2. Transportation of Flower Bulbs 3. Forcing and Greenhouse Technology V. MAJOR FLOWER BULB ORGANIZATIONS A. -

Dyuhei Sato Division of Genetics, Bot. Inst. Faculty of Science, Tokyo
ANALYSIS OF THE KARYOTYPES IN YUCCA, A GA VE AND THE RELATED GENERA WITH SPECIAL REFERENCE TO THE PHYLOGENETIC SIGNIFICANCEI~ Dyuhei SATo Divisionof Genetics, Bot. Inst. Faculty of Science, Tokyo Imperial University McKelvey and Sax (2933) have called attention to the existence of taxonomic and cytological similarities of the genera Yucca, Hesperoyucca, Gleistvucca,Hesperoaloe and Samuela of the Liliaceae with the genera Agave and Fourcroya which belong to a related family, Amaryllidaceae. Wh.itaker (1934) also has reported that Polianhes and Fourcroya have exactly the same chromosome constitution as the Yucca-Abave karyotype (5 long and 25 short chromosomes) (Figs. 1, 2). These observations when considered in respect to taxonomic resemblances, seem to indicate that the genera mentioned above are more closely related than it is shown by their classifica- tion into distinct families. Whitaker also has remarked that Dasylirion (2n=38) and ATolina(2n=36) in Yucceae and Doryanthes (2n=36) in Agavoideae are of different karyotypes from the Yucca-Agave type. In the present work an analysis of the karyotypes in Liliaceous plants has been attempted and several karyotypes have been found in Scilloideae. Eucornis and Carassia have been selected with the purpose of discovering a possible connecting link between these genera and the Yucca-Agave group. In the present paper an analysis of the karyotypes of the following species is given. LILIACEAE Scilloideae 211 Fig. Euconis undulata 60=8L+8M+44S (4b)2) 3 Euconsispallidi ora 60=8L+8M+44S (4b) 4 Eucomispunctata 60=8L±8M+44S (4b) 5 Camassiaescrema 30=6L+24S (2b) 6 Yucceae Yuccafilamentosa 30 60=1OL+50S (2b) 1, 7 Yuccarecurvifolia 30 60=1OL+50S (2b) 2, 8 Yuccaaloifolia 60=1OL+50S (2b) 9 „ var. -

~Gazine American Horticultural
'I'IIE ~ERICA.N ~GAZINE AMERICAN HORTICULTURAL 1600 BLADENSBURG ROAD, NORTHEAST. WASHINGTON, D. C. For United Horticulture *** to accumulate, increase, and disseminate horticultural information Editorial Committee Directors JOHN L. CREECH, Chairman Terms Expiring 1964 R. C. ALLEN W. H. HODGE Ohio P. H. BRYDON FREDERIC P. LEE California CONRAD B. LINK CARL "V. FENNINGER Pennsylvania CURTIS MAY JOHN E. GRAF District of Columbia FREDERICK G. MEYER GRACE P. WILSON Mm'yland WILBUR H. YOUNGMAN Terms Expiring 1965 HAROLD EpSTEIN New York Officers FRED C. GALLE Georgia PRESIDENT FRED J. NISBET North Carolina RUSSELL J. SEIBERT J. FRANKLIN STYER Kennett Square, Pennsylvania Pennsylvania DONALD WYMAN FIRST VICE-PRESIDENT Massachusetts RAy C. ALLEN Terms Expiring 1966 Mansfield, Ohio J. HAROLD CLARKE Washington SECOND VICE-PRESIDENT J AN DE GRAAFF MRS. JULIAN W. HILL Oregon Wilmington, Delaware CARLTON B. LEES Massachusetts RUSSELL J. SEIBERT ACTING SECRETARY'TREASURER . Pennsylvania GRACE P. WILSON DONALD WATSQN Bladensburg, Maryland Michigan The American Horticultural Magazine is the official publication of the American Horticultural Society and is issued four times a year during the quarters commencing with January, April, July and October. It is devoted to thCl dissemination of knowledge in the science and art of growing ornamental plants, fruits, vegetables, and related subjects. Original papers increasing the historical, varietal, and cultural knowledges of plant materials of economic and aesthetic importance are welcomed and will be published as early as possible. The Chairman of the Editorial Committee should be consulted for manuscript specifications. Reprints will be furnished in accordance with the following schedule of prices, plus post age, and should be ordered at the time the galley proof is returned by the author: One hunclred copies-2 pp $6.60; 4 pp $12.10; 8 pp $25.30; 12 pp $36.30; Covers $12.10. -

Rutgers Home Gardeners School: the Beauty of Bulbs
The Beauty of Bulbs Bruce Crawford March 17, 2018 Director, Rutgers Gardens Rutgersgardens.rutgers.edu In general, ‘bulbs’, or more properly, geophytes are easy plants to grow, requiring full sun, good drainage and moderately fertile soils. Geophytes are defined as any non-woody plant with an underground storage organ. These storage organs contain carbohydrates, nutrients and water and allow the plant to endure extended periods of time that are not suitable for plant growth. Types of Geophytes include: Bulb – Swollen leaves or leaf stalks, attached at the bottom to a modified stem called a basal plant. The outer layers are modified leaves called scales. Scales contain necessary foods to sustain the bulb during dormancy and early growth. The outermost scales become dry and form a papery covering or tunic. At the center are developed, albeit embryonic flowers, leaves and stem(s). Roots develop from the basal plate. Examples are Tulipia (Tulip), Narcissus (Daffodil), and Allium (Flowering Onion). Corm – A swollen stem that is modified for food storage. Eyes or growing points develop on top of the corm. Roots develop from a basal plate on the bottom of the corm, similar to bulbs. The dried bases of the leaves from an outer layer, also called the tunic. Examples include Crocus and Erythronium (Dog Tooth Violet). Tuber – Also a modified stem, but it lacks a basal plate and a tunic. Roots, shoots and leaves grow from eyes. Examples are Cyclamen, Eranthis (Winter Aconite) and Anemone (Wind Flower). Tuberous Roots – These enlarged storage elements resemble tubers but are swollen roots, not stems. During active growth, they produce a fibrous root system for water and nutrient absorption. -

Ornithogalum L
Sistemática del género Ornithogalum L. (Hyacinthaceae) en el Mediterráneo occidental: implicaciones taxonómicas, filogenéticas y biogeográficas Mario Martínez Azorín Departamento de Ciencias Ambientales y Recursos Naturales Sistemática del género Ornithogalum L. (Hyacinthaceae) en el Mediterráneo occidental: implicaciones taxonómicas, filogenéticas y biogeográficas Tesis Doctoral Mario Martínez Azorín Abril 2008 Departamento de Ciencias Ambientales y Recursos Naturales MANUEL B. CRESPO VILLALBA, CATEDRÁTICO DE UNIVERSIDAD, Y ANA JUAN GALLARDO, PROFESORA CONTRATADA DOCTORA, MIEMBROS DEL ÁREA DE BOTÁNICA DEL DEPARTAMENTO DE CIENCIAS AMBIENTALES Y RECURSOS NATURALES, Y DEL I.U. CIBIO, DE LA UNIVERSIDAD DE ALICANTE, C E R T I F I C A N: Que la presente memoria titulada “Sistemática del género Ornithogalum L. (Hyacinthaceae) en el Mediterráneo occidental: implicaciones taxonómicas, filogenéticas y biogeográficas”, que para aspirar al grado de Doctor presenta el Licenciado en Biología D. Mario Martínez Azorín, ha sido realizada bajo nuestra dirección en este Departamento, y considerando que reúne los requisitos necesarios a tal efecto, autorizamos que siga los trámites preceptivos para su exposición y defensa. Y para que así conste donde convenga al interesado, y a petición suya, expedimos el presente certificado en Alicante, a veinticinco de febrero de dos mil ocho. A mis padres, Encarna y Antonio a Adrian y a Almudena Agradecimientos AGRADECIMIENTOS Ante todo, deseo agradecer a todas las personas que con su ayuda, apoyo y comprensión han hecho posible este trabajo. En primer lugar, quiero dar mi más sincero agradecimiento a mis directores de Tesis, los Drs. Manuel B. Crespo Villalba y Ana Juan Gallardo, por haberme animado a emprender el largo camino que culmina con la realización del presente trabajo. -
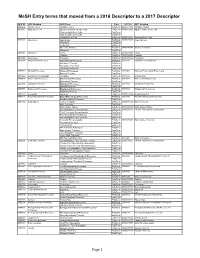
Mesh Entry Terms That Moved from a 2016 Descriptor to a 2017 Descriptor
MeSH Entry terms that moved from a 2016 Descriptor to a 2017 Descriptor 2016 UI 2016 Heading 2016 Term Type 2017 UI 2017 Heading D000204 Activity Cycles Ultradian Cycles EntryTerm D000071218 Ultradian Rhythm D053687 Adult Stem Cells Spermatogonial Progenitor Cells EntryTerm D000072956 Adult Germline Stem Cells Germline Adult Stem Cells EntryTerm Adult Germline Stem Cells EntryTerm Oogonial Stem Cells EntryTerm D000072977 Oogonial Stem Cells D027881 Agavaceae Agavaceae PrefTerm D000070357 Asparagaceae Polianthes EntryTerm Hesperaloe EntryTerm Flax, New Zealand EntryTerm D000070380 Xanthorrhoeaceae Phormium EntryTerm D000383 Agriculture Farms EntryTerm D000072480 Farms Gardens EntryTerm D000072506 Gardens D019659 Asteraceae Psacalium EntryTerm D000070216 Psacalium D055588 Astronomical Processes Astronomical Processes PrefTerm D055580 Astronomical Phenomena Astronomic Process EntryTerm Astronomic Processes EntryTerm Astronomical Process EntryTerm D055683 Bacterial Processes Bacterial Processes PrefTerm D018407 Bacterial Physiological Phenomena Bacterial Process EntryTerm D001626 Bethanechol Compounds Urecholine EntryTerm D018723 Bethanechol D055438 Biochemical Processes Biochemical Processes PrefTerm D001669 Biochemical Phenomena Biochemical Process EntryTerm D055694 Biological Processes Biological Processes PrefTerm D001686 Biological Phenomena Biological Process EntryTerm D055597 Biophysical Processes Biophysical Processes PrefTerm D055592 Biophysical Phenomena Biophysical Process EntryTerm D001727 Bisexuality Bisexuals EntryTerm D000072339 -

Threats to Australia's Grazing Industries by Garden
final report Project Code: NBP.357 Prepared by: Jenny Barker, Rod Randall,Tony Grice Co-operative Research Centre for Australian Weed Management Date published: May 2006 ISBN: 1 74036 781 2 PUBLISHED BY Meat and Livestock Australia Limited Locked Bag 991 NORTH SYDNEY NSW 2059 Weeds of the future? Threats to Australia’s grazing industries by garden plants Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. Weeds of the future? Threats to Australia’s grazing industries by garden plants Abstract This report identifies 281 introduced garden plants and 800 lower priority species that present a significant risk to Australia’s grazing industries should they naturalise. Of the 281 species: • Nearly all have been recorded overseas as agricultural or environmental weeds (or both); • More than one tenth (11%) have been recorded as noxious weeds overseas; • At least one third (33%) are toxic and may harm or even kill livestock; • Almost all have been commercially available in Australia in the last 20 years; • Over two thirds (70%) were still available from Australian nurseries in 2004; • Over two thirds (72%) are not currently recognised as weeds under either State or Commonwealth legislation. -

Una Nueva Especie De Tanqua Karoo (Sudáfrica), Africa), with Notes on E
2311_Ethesia_Mnez.Azo.af_Anales 69(2).qxd 14/12/2012 12:38 Página 201 Anales del Jardín Botánico de Madrid 69(2): 201-208, julio-diciembre 2012. ISSN: 0211-1322. doi: 10.3989/ajbm. 2311 Ethesia tanquana (Ornithogaloideae, Hyacinthaceae), a new species from the Tanqua Karoo (South Africa), with notes on E. haalenbergensis Mario Martínez-Azorín* & Manuel B. Crespo CIBIO (Instituto de la Biodiversidad), Universidad de Alicante, Apartado 99, E-03080 Alicante, Spain; [email protected] Abstract Resumen Martínez-Azorín, M. & Crespo, M.B. 2012. Ethesia tanquana (Ornithoga- Martínez-Azorín, M. & Crespo, M.B. 2012. Ethesia tanquana (Ornithoga- loideae, Hyacinthaceae), a new species from the Tanqua Karoo (South loideae, Hyacinthaceae), una nueva especie de Tanqua Karoo (Sudáfrica), Africa), with notes on E. haalenbergensis. Anales Jard. Bot. Madrid 69(2): con notas sobre E. haalenbergensis. Anales Jard. Bot. Madrid 69(2): 201- 201-208. 208 (en inglés). As a part of a taxonomic revision of Ethesia Raf., a new species, E. tanqua- En el marco de la revisión taxonómica de Ethesia Raf., se describe una nue- na Mart.-Azorín & M.B.Crespo, is described from the Tanqua Karoo in va especie, E. tanquana Mart.-Azorín & M.B.Crespo, del Tanqua Karoo en South Africa. This new species is at first sight similar to E. haalenbergensis Sudáfrica. Esta nueva especie se asemeja a primera vista a E. haalenber- (U.Müll.-Doblies & D.Müll.-Doblies) Mart.-Azorín, M.B.Crespo & Juan and gensis (U.Müll.-Doblies & D.Müll.-Doblies) Mart.-Azorín, M.B.Crespo & also E. xanthochlora (Baker) Mart.-Azorín, M.B.Crespo & Juan, but it dif- Juan y E. -

April 1964 AMERICAN HORTICULTURAL
TIIE .A.~ERIC.A.N ~GAZINE April 1964 AMERICAN HORTICULTURAL 1600 BLADENSBURG ROAD, NORTHEAST. WASHINGTON, D. C. For United Horticulture *** to accumulate, increase, and disseminate horticultural information Editorial Committee Directors Terms Expiring 1964 JOHN L. CREECH, Chairman R. C. ALLEN W. H . HODGE Ohio P. H. BRYDON FREDERIC P. LEE California CARL W. FENNINGER CONRAD B . LINK Pennsylvania CURTIS MAY JOHN E . GRAF District of Columbia FREDERICK G . MEYER GRACE P. WILSON Maryland WILBUR H . YOUNGMAN Terms Expiring 1965 HAROLD EpSTEIN New YOI'k Officers FRED C . GALLE Georgia PRESIDENT FRED J. NISBET North Carolina R USSELL J. SEIBERT J. FRANKLIN STYER Kennett Square, Pennsylvania Pennsylvania DONALD WYMAN FIRST VICE-PRESIDENT Massachusetts RAy C . ALLEN Terms Expiring 1966 Mansfie ld, Ohio J. HAROLD CLARKE Washington SECOND VICE-PRESIDENT JAN DE GRAAFF MRS. JULIAN W. HILL Oregon Wilm ington, Delaware CARLTON B . LEES Massachusetts RUSSELL J. SEIBERT ACTING SECRETARY-TREASURER . Pennsylvania GRACE P. WILSON DONALD WATSON Bladensburg, Maryland Michigan The American Horticultural Magazine is the official publication of the American Horticultural Society and is issued four times a year during the quarters commencing with January, April, J~ly and October. It is devoted to the dissemination of knowledge in the science and art of growmg ornamental plants, fruits, vegetables, and related subjects. Original papers increasing the historical, varietal, and cultural know ledges of plant mate~ials of economic and aesthetic importance are welcomed and will be published as early as possible. The Chairman of the Editorial Committee should be consulted for manuscript specifications. Reprints will be furnished in accordance with the following schedule of prices, plus post age, and should be ordered at the time the galley proof is returned by the author: One hundred copies-2 pp $6.60; 4 pp $12.10; 8 pp $25.30; 12 pp $36.30; Covers $12.10. -

NATIONAL HORTICULTURE POLICY June 2012
REPUBLIC OF KENYA NATIONAL HORTICULTURE POLICY June 2012 © 2012. Government of Kenya Agricultural Sector Coordination Unit (ASCU) Kilimo House, Cathedral Road PO Box 30028–00100. Nairobi, Kenya Tel: +254 20 2046856. Fax: +254 20 2046985 Email: [email protected]. website: www.ascu.go.ke Contents Contents.........................................................................................................................iii Foreword......................................................................................................................v Preface..........................................................................................................................vii Acknowledgement..........................................................................................................viii Executive Summary.....................................................................................................ix Abbreviations and Acronyms.....................................................................................xi 1. IntRoDUCtIon................................................................................................1 1.1 Background............................................................................................................1 1.2 The Role of Horticulture in the Kenyan Economy .............................................1 1.3 Justification..........................................................................................................8 1.4 Objectives....................................................................................