Eukaryotic-Like Ribosomal RNA Region in Lokiarchaeota 2 Authors: Petar I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Domains of Life, Not Three?
Asgard Archaea Two domains of life, not three? A picture of visual interpretation of the Wagner’s opera Das Rheingold, a part of Richard Wagner’s Der Ring des Nibelungen Loki's Castle is a field of five active hydrothermal vents in the mid-Atlantic Ocean, located at 73 degrees north on the Mid-Atlantic Ridge between Greenland and Norway at a depth of 2,352 metres The vents were discovered in 2008 by a multinational scientific expedition of the university of Bergen, and are the most northerly black smokers to date. The five active chimneys of Loki's Castle are venting water as hot as 300 °C and sit on a vast mound of sulfide minerals. The vent field was given the name Loki's Castle as its shape reminded its discoverers of a fantasy castle. The reference is to the ancient Norse god of trickery, Loki. The top three feet (1 m) of a vent chimney almost 40 feet (12 m) tall at Loki's Castle in mid-July 2008. Visible at left is the arm of a remotely operated vehicle, reaching in to take fluid samples. Loki’s Castle Wents Loki's Castle - a field of five active hydrothermal vents in the mid-Atlantic Ocean - at 73 degrees north on the Mid-Atlantic Ridge - at a depth of 2,352 meters In Norse mythology, Loki is a cunning trickster who has the ability to change his shape and sex. Loki is represented as the companion of the great gods Odin and Thor. Preliminary observations have shown the warm area around the Loki's Castle vents to be alive with diverse and apparently unique microorganisms, unlike vent communities observed elsewhere. -
Lokiarchaeota: Biologists Discover 'Missing Link' Microorganism
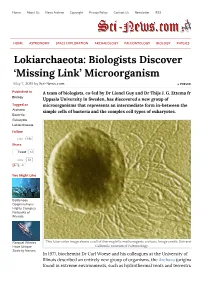
Home About Us News Archive Copyright Privacy Policy Contact Us Newsletter RSS HOME ASTRONOMY SPACE EXPLORATION ARCHAEOLOGY PALEONTOLOGY BIOLOGY PHYSICS Lokiarchaeota: Biologists Discover ‘Missing Link’ Microorganism May 7, 2015 by Sci-News.com « PREVIOUS Published in A team of biologists, co-led by Dr Lionel Guy and Dr Thijs J. G. Ettema from Biology Uppsala University in Sweden, has discovered a new group of Tagged as microorganisms that represents an intermediate form in-between the Archaea simple cells of bacteria and the complex cell types of eukaryotes. Bacteria Eukaryote Lokiarchaeota Follow Like 16k Share Tweet 12 Like 58 41 You Might Like Bottlenose Dolphins Form Highly Complex Networks of Friends Rorqual Whales This false-color image shows a cell of thermophilic methanogenic archaea. Image credit: University of Have Unique California Museum of Paleontology. Stretchy Nerves In 1977, biochemist Dr Carl Woese and his colleagues at the University of Illinois described an entirely new group of organisms, the Archaea (originally found in extreme environments, such as hydrothermal vents and terrestrial hot springs). The scientists were studying relationships among the prokaryotes using DNA Extinction of sequences, and found that Archaea have distinct molecular characteristics World’s Largest separating them from bacteria as well as from eukaryotes. They proposed that Herbivores May Lead to Empty life can be divided into three domains: Eukaryota, Eubacteria, and Landscapes, Say Archaebacteria. Researchers Despite that archaeal cells were simple and small like bacteria, scientists found that Archaea were more closely related to organisms with complex cell types, a group collectively known as ‘eukaryotes.’ This observation has puzzled Sichuan Bush biologists for years. -

Origin and Evolution of Eukaryotic Compartmentalization
TESI DOCTORAL UPF 2016 Origin and evolution of eukaryotic compartmentalization Alexandros Pittis Director Dr. Toni Gabaldon´ Comparative Genomics Group Bioinformatics and Genomics Department Centre for Genomic Regulation (CRG) To my father Stavros for the PhD he never did Acknowledgments Looking back at the years of my PhD in the Comparative Genomics group at CRG, I feel it was equally a process of scientific, as well as personal development. All this time I was very privileged to be surrounded by people that offered me their generous support in both fronts. And they were available in the very moments that I was fighting more myself than the unsolvable (anyway) comparative genomics puzzles. I hope that in the future I will have many times the chance to express them my appreciation, way beyond these few words. First, to Toni, my supervisor, for all his support, and patience, and confidence, and respect, especially at the moments that things did not seem that promising. He offered me freedom, to try, to think, to fail, to learn, to achieve that few enjoy during their PhD, and I am very thankful to him. Then, to my good friends and colleagues, those that I found in the group already, and others that joined after me. A very special thanks to Marinita and Jaime, for all their valuable time, and guidance and paradigm, which nevertheless I never managed to follow. They have both marked my phylogenomics path so far and I cannot escape. To Les and Salvi, my fellow students and pals at the time for all that we shared; to Gab and Fran and Dam, for -

Spatial Separation of Ribosomes and DNA in Asgard Archaeal Cells ✉ ✉ Burak Avcı 1,2 , Jakob Brandt3, Dikla Nachmias4, Natalie Elia4, Mads Albertsen 3, Thijs J
www.nature.com/ismej BRIEF COMMUNICATION OPEN Spatial separation of ribosomes and DNA in Asgard archaeal cells ✉ ✉ Burak Avcı 1,2 , Jakob Brandt3, Dikla Nachmias4, Natalie Elia4, Mads Albertsen 3, Thijs J. G. Ettema 2, Andreas Schramm1,5 and Kasper Urup Kjeldsen1 © The Author(s) 2021 The origin of the eukaryotic cell is a major open question in biology. Asgard archaea are the closest known prokaryotic relatives of eukaryotes, and their genomes encode various eukaryotic signature proteins, indicating some elements of cellular complexity prior to the emergence of the first eukaryotic cell. Yet, microscopic evidence to demonstrate the cellular structure of uncultivated Asgard archaea in the environment is thus far lacking. We used primer-free sequencing to retrieve 715 almost full-length Loki- and Heimdallarchaeota 16S rRNA sequences and designed novel oligonucleotide probes to visualize their cells in marine sediments (Aarhus Bay, Denmark) using catalyzed reporter deposition-fluorescence in situ hybridization (CARD-FISH). Super-resolution microscopy revealed 1–2 µm large, coccoid cells, sometimes occurring as aggregates. Remarkably, the DNA staining was spatially separated from ribosome-originated FISH signals by 50–280 nm. This suggests that the genomic material is condensed and spatially distinct in a particular location and could indicate compartmentalization or membrane invagination in Asgard archaeal cells. The ISME Journal; https://doi.org/10.1038/s41396-021-01098-3 INTRODUCTION cells in marine sediments [8] yet methodical limitations did not The origin of the eukaryotic cell is a major unresolved puzzle in allow to discern single cells and to draw any in-depth conclusion the history of life. -

Archaea and the Origin of Eukaryotes
REVIEWS Archaea and the origin of eukaryotes Laura Eme, Anja Spang, Jonathan Lombard, Courtney W. Stairs and Thijs J. G. Ettema Abstract | Woese and Fox’s 1977 paper on the discovery of the Archaea triggered a revolution in the field of evolutionary biology by showing that life was divided into not only prokaryotes and eukaryotes. Rather, they revealed that prokaryotes comprise two distinct types of organisms, the Bacteria and the Archaea. In subsequent years, molecular phylogenetic analyses indicated that eukaryotes and the Archaea represent sister groups in the tree of life. During the genomic era, it became evident that eukaryotic cells possess a mixture of archaeal and bacterial features in addition to eukaryotic-specific features. Although it has been generally accepted for some time that mitochondria descend from endosymbiotic alphaproteobacteria, the precise evolutionary relationship between eukaryotes and archaea has continued to be a subject of debate. In this Review, we outline a brief history of the changing shape of the tree of life and examine how the recent discovery of a myriad of diverse archaeal lineages has changed our understanding of the evolutionary relationships between the three domains of life and the origin of eukaryotes. Furthermore, we revisit central questions regarding the process of eukaryogenesis and discuss what can currently be inferred about the evolutionary transition from the first to the last eukaryotic common ancestor. Sister groups Two descendants that split The pioneering work by Carl Woese and colleagues In this Review, we discuss how culture- independent from the same node; the revealed that all cellular life could be divided into three genomics has transformed our understanding of descendants are each other’s major evolutionary lines (also called domains): the archaeal diversity and how this has influenced our closest relative. -

Newly Found Microbe Is Close Relative of Complex Life - BBC News
Newly found microbe is close relative of complex life - BBC News http://www.bbc.com/news/science-environment-32610177 Cookies on the BBC website We use cookies to ensure that we give you the best experience on our website. We also use cookies to ensure we show you advertising that is relevant to you. If you continue without changing your settings, we'll assume that you are happy to receive all cookies on the BBC website. However, if you would like to, you can change your cookie settings at any time. Continue Change settings Find out more News Sport Weather Shop Earth More Home Video World UK Business Tech Science Magazine Entertainment & Arts Health In PicturesMore ADVERTISEMENT Science & Environment By Paul Rincon Science editor, BBC News website 6 May 2015 Science & Environment The new group of archaea was discovered in sediments along the Arctic Mid-Ocean Ridge A newly discovered life form could help resolve one of the most contentious conundrums in modern biology. All organisms on Earth are classified as either prokaryotes, which have simple cells, or eukaryotes, which have larger, more complex cells. But the two cell types are so divergent that understanding how one evolved from the other has foxed biologists. The new microbes, reported in Nature journal, go some way to bridging that gap. They have been named Lokiarchaeota, partly after the Loki's Castle volcanic vent system lying 15km away from the site where the microbes' genetic material was isolated in cold marine sediments of the Arctic Mid-Ocean Ridge. 09.05.2015 14:32 Newly found microbe is close relative of complex life - BBC News http://www.bbc.com/news/science-environment-32610177 Domain names Prokaryotes are single-celled organisms and comprise all bacteria and archaea (a group of microbes that were once considered to be bacteria, but now form a separate domain of life). -

Evidence Supporting a Viral Origin of the Eukaryotic Nucleus
bioRxiv preprint doi: https://doi.org/10.1101/679175; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Evidence supporting a viral origin of the eukaryotic nucleus 2 3 4 Dr Philip JL Bell 5 Microbiogen Pty Ltd 6 Correspondence should be addressed to Email [email protected] 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Keywords: Viral eukaryogenesis, nucleus, eukaryote origin, viral factory, mRNA capping, phylogeny 26 27 1 bioRxiv preprint doi: https://doi.org/10.1101/679175; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 28 Abstract 29 30 The defining feature of the eukaryotic cell is the possession of a nucleus that uncouples transcription 31 from translation. This uncoupling of transcription from translation depends on a complex process 32 employing hundreds of eukaryotic specific genes acting in concert and requires the 7- 33 methylguanylate (m7G) cap to prime eukaryotic mRNA for splicing, nuclear export, and cytoplasmic 34 translation. The origin of this complex system is currently a paradox since it is not found or needed 35 in prokaryotic cells which lack nuclei, yet it was apparently present and fully functional in the Last 36 Eukaryotic Common Ancestor (LECA). -

A Scientific Literature Review Comic by Lilja Strang
A scientific literature review comic by Lilja Strang In Norse mythology all of life is contained within nine realms. These realms are connected by Yggdrasil, or the World Tree. A similar concept exists in biology. It’s called the tree of life and it shows the evolutionary connections between all life on earth. These two trees have more in common than most people think. To begin our story, think back to your earliest biology class. You probably saw either the 1959 tree of life, organized into Carl Woese—1990 either 5 kingdoms, or the 1990 tree with 3 domains. The 5 kingdoms tree compared life on the basis of how things looked. Microscopic organisms were too small to see easily, and were excluded. Whittaker’s 5 Kingdom Tree (1959) Woese’s 3 Domain Tree (1990) Plantae Fungi Animalia Bacteria Archaea Eucarya Carl Woese’s tree Protista was based on the best DNA evidence Monera of his time. Here, the microscopic Bacteria and Archaea were front and center. In fact, Woese realized that the Archaea were Níðhöggr (Nidhogg) is the dragon that gnaws at the roots of Yggdrasil. very special. In the 1990s, Archaea were thought to only be extremophiles living in very Methanosarcina mazeii produces the methane gas in cow farts. hot/cold, acidic/basic, or There are no known methane otherwise unusual habitats. producing Bacteria, only Archaea. Sulfolobus live in the Geysers of Yellowstone at temperatures of 40–55°C (104–131°F). Methane producers are found in humans too. Ferroplasma acidiphilum live They’re similar to those in the toxic byproducts of found in cows. -

Genomic and Evolutionary Exploration of Asgard Archaea
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1861 Genomic and evolutionary exploration of Asgard archaea EVA F. CACERES ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0761-9 UPPSALA urn:nbn:se:uu:diva-393710 2019 Dissertation presented at Uppsala University to be publicly examined in B22, Biomedical Center (BMC), Husargatan 3, Uppsala, Thursday, 14 November 2019 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Simonetta Gribaldo (Institut Pasteur, Department of Microbiology). Abstract Caceres, E. F. 2019. Genomic and evolutionary exploration of Asgard archaea. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1861. 88 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0761-9. Current evolutionary theories postulate that eukaryotes emerged from the symbiosis of an archaeal host with, at least, one bacterial symbiont. However, our limited grasp of microbial diversity hampers insights into the features of the prokaryotic ancestors of eukaryotes. This thesis focuses on the study of a group of uncultured archaea to better understand both existing archaeal diversity and the origin of eukaryotes. In a first study, we used short-read metagenomic approaches to obtain eight genomes of Lokiarchaeum relatives. Using these data we described the Asgard superphylum, comprised of at least four different phyla: Lokiarchaeota, Odinarchaeota, Thorarchaeota and Heimdallarchaoeta. Phylogenetic analyses suggested that eukaryotes affiliate with the Asgard group, albeit the exact position of eukaryotes with respect to Asgard archaea members remained inconclusive. Comparative genomics showed that Asgard archaea genomes encoded homologs of numerous eukaryotic signature proteins (ESPs), which had never been observed in Archaea before. -

Lokiarchaea Are Close Relatives of Euryarchaeota, Not
Lokiarchaea are close relatives of Euryarchaeota, not bridging the gap between prokaryotes and eukaryotes Violette da Cunha, Morgan Gaia, Danièle Gadelle, Arshan Nasir, Patrick Forterre To cite this version: Violette da Cunha, Morgan Gaia, Danièle Gadelle, Arshan Nasir, Patrick Forterre. Lokiarchaea are close relatives of Euryarchaeota, not bridging the gap between prokaryotes and eukaryotes. PLoS Ge- netics, Public Library of Science, 2017, 13 (6), pp.e1006810. 10.1371/journal.pgen.1006810. pasteur- 01570207 HAL Id: pasteur-01570207 https://hal-pasteur.archives-ouvertes.fr/pasteur-01570207 Submitted on 28 Jul 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License RESEARCH ARTICLE Lokiarchaea are close relatives of Euryarchaeota, not bridging the gap between prokaryotes and eukaryotes Violette Da Cunha1,2³, Morgan Gaia1³, Daniele Gadelle2, Arshan Nasir3, Patrick Forterre1,2* 1 Institut Pasteur, Unite de Biologie MoleÂculaire du Gène chez les Extrêmophiles (BMGE), DeÂpartement de Microbiologie Paris, France, 2 Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ. Paris- Sud, Universite Paris-Saclay, Gif-sur-Yvette cedex, France, 3 Department of Biosciences, COMSATS a1111111111 Institute of Information Technology, Islamabad, Pakistan a1111111111 ³ These authors share first authorship on this work. -

Lokiarchaeota Marks the Transition Between the Archaeal and Eukaryotic Selenocysteine Encoding Systems
Lokiarchaeota Marks the Transition between the Archaeal and Eukaryotic Selenocysteine Encoding Systems The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Mariotti, Marco, Alexei V. Lobanov, Bruno Manta, Didac Santesmasses, Andreu Bofill, Roderic Guigó, Toni Gabaldón, and Vadim N. Gladyshev. 2016. “Lokiarchaeota Marks the Transition between the Archaeal and Eukaryotic Selenocysteine Encoding Systems.” Molecular Biology and Evolution 33 (9): 2441-2453. doi:10.1093/molbev/msw122. http://dx.doi.org/10.1093/molbev/ msw122. Published Version doi:10.1093/molbev/msw122 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:29002393 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Lokiarchaeota Marks the Transition between the Archaeal and Eukaryotic Selenocysteine Encoding Systems Marco Mariotti,1,2,3 Alexei V. Lobanov,1 Bruno Manta,1 Didac Santesmasses,2,3 Andreu Bofill,2,3 Roderic Guigo, 2,3 Toni Gabaldon, 2,3,4 and Vadim N. Gladyshev*,1 1Division of Genetics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 2Bioinformatics and Genomics Programme, Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain 3Universitat Pompeu Fabra (UPF); and Institut Hospital del Mar d’Investigacions Me`diques (IMIM), Barcelona, Spain 4Institucio Catalana de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain *Corresponding author: E-mail: [email protected]. -

Undinarchaeota Illuminate DPANN Phylogeny and the Impact of Gene Transfer on Archaeal Evolution
ARTICLE https://doi.org/10.1038/s41467-020-17408-w OPEN Undinarchaeota illuminate DPANN phylogeny and the impact of gene transfer on archaeal evolution Nina Dombrowski 1, Tom A. Williams 2, Jiarui Sun3, Benjamin J. Woodcroft 3, Jun-Hoe Lee 4, ✉ Bui Quang Minh 5, Christian Rinke 3 & Anja Spang 1,4 The recently discovered DPANN archaea are a potentially deep-branching, monophyletic radiation of organisms with small cells and genomes. However, the monophyly and early 1234567890():,; emergence of the various DPANN clades and their role in life’s evolution are debated. Here, we reconstructed and analysed genomes of an uncharacterized archaeal phylum (Candidatus Undinarchaeota), revealing that its members have small genomes and, while potentially being able to conserve energy through fermentation, likely depend on partner organisms for the acquisition of certain metabolites. Our phylogenomic analyses robustly place Undinarchaeota as an independent lineage between two highly supported ‘DPANN’ clans. Further, our ana- lyses suggest that DPANN have exchanged core genes with their hosts, adding to the dif- ficulty of placing DPANN in the tree of life. This pattern can be sufficiently dominant to allow identifying known symbiont-host clades based on routes of gene transfer. Together, our work provides insights into the origins and evolution of DPANN and their hosts. 1 NIOZ, Royal Netherlands Institute for Sea Research, Department of Marine Microbiology and Biogeochemistry, and Utrecht University, P.O. Box 59 , NL- 1790 AB Den Burg, The Netherlands. 2 School of Biological Sciences, University of Bristol, Bristol BS8 1TQ, UK. 3 Australian Centre for Ecogenomics, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD 4072, Australia.