Natural History of Ferns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ferns Robert H
Southern Illinois University Carbondale OpenSIUC Illustrated Flora of Illinois Southern Illinois University Press 10-1999 Ferns Robert H. Mohlenbrock Southern Illinois University Carbondale Follow this and additional works at: http://opensiuc.lib.siu.edu/siupress_flora_of_illinois Part of the Botany Commons Recommended Citation Mohlenbrock, Robert H., "Ferns" (1999). Illustrated Flora of Illinois. 3. http://opensiuc.lib.siu.edu/siupress_flora_of_illinois/3 This Book is brought to you for free and open access by the Southern Illinois University Press at OpenSIUC. It has been accepted for inclusion in Illustrated Flora of Illinois by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. THE ILLUSTRATED FLORA OF ILLINOIS ROBERT H. MOHLENBROCK, General Editor THE ILLUSTRATED FLORA OF ILLINOIS s Second Edition Robert H. Mohlenbrock SOUTHERN ILLINOIS UNIVERSITY PRESS Carbondale and Edwardsville COPYRIGHT© 1967 by Southern Illinois University Press SECOND EDITION COPYRIGHT © 1999 by the Board of Trustees, Southern Illinois University All rights reserved Printed in the United States of America 02 01 00 99 4 3 2 1 Library of Congress Cataloging-in-Publication Data Mohlenbrock, Robert H., 1931- Ferns I Robert H. Mohlenbrock. - 2nd ed. p. em.- (The illustrated flora of Illinois) Includes bibliographical references and index. 1. Ferns-Illinois-Identification. 2. Ferns-Illinois-Pictorial works. 3. Ferns-Illinois-Geographical distribution-Maps. 4. Botanical illustration. I. Title. II. Series. QK525.5.I4M6 1999 587'.3'09773-dc21 99-17308 ISBN 0-8093-2255-2 (cloth: alk. paper) CIP The paper used in this publication meets the minimum requirements of American National Standard for Information Sciences-Permanence of Paper for Printed Library Materials, ANSI Z39.48-1984.§ This book is dedicated to Miss E. -

Journal the New York Botanical Garden
VOL. XXXV SEPTEMBER, 1934 No. 417 JOURNAL OF THE NEW YORK BOTANICAL GARDEN FERNS WITHIN ONE HUNDRED MILES OF NEW YORK CITY JOHN K. SMALL TRIFOLIUM VIRGINICUM IN CULTIVATION T. H. EVERETT THE ELIZABETH GERTRUDE BRITTON MOSS HERBARIUM IS ESTABLISHED E. D. MERRILL SCIENCE COURSE FOR PROFESSIONAL GARDENERS ENTERS THIRD YEAR PUBLIC LECTURES SCHEDULED FOR SEPTEMBER, OCTOBER, AND NOVEMBER A GLANCE AT CURRENT LITERATURE CAROL H. WOODWARD NOTES, NEWS, AND COMMENT PUBLISHED FOR THE GARDEN AT LIME AND GREEN STREETS, LANCASTER, PA. THE SCIENCE PRESS PRINTING COMPANY Entered at the post-office in Lancaster, Pa., as second-class matter. Annual subscription $1.00 Single copies 10 cents Free to members of the Garden THE NEW YORK BOTANICAL GARDEN BOARD OF MANAGERS I. ELECTIVE MANAGERS Until 1935: L. H. BAILEY, THOMAS J. DOLEN, MARSHALL FIELD, MRS. ELON HUNTINGTON HOOKER, KENNETH K. MACKENZIE, JOHN L. MERRILL (Vice-presi dent and Treasurer), and H. HOBART PORTER. Until 1936: ARTHUR M. ANDERSON, HENRY W. DE FOREST (President), CLARENCE LEWIS, E. D. MERRILL (Director and Secretary), HENRY DE LA MON TAGNE, JR. (Assistant Treasurer & Business Manager), and LEWIS RUTHER- FURD MORRIS. Until 1937: HENRY DE FOREST BALDWIN (Vice-president), GEORGE S. BREWSTER, CHILDS FRICK, ADOLPH LEWISOHN, HENRY LOCKHART, JR., D. T. MACDOUGAL, and JOSEPH R. SWAN. II. EX-OFFICIO MANAGERS FIORELLO H. LAGUARDIA, Mayor of the City of New York. ROBERT MOSES, Park Commissioner. GEORGE J. RYAN, President of the Board of Education. III. APPOINTIVE MANAGERS A. F. BLAKESLEE, appointed by the Torrey Botanical Club. R. A. HARPER, SAM F. TRELEASE, EDMUND W. SINNOTT, and MARSTON T. -

Piedmont Cliff (Acidic Subtype)
PIEDMONT CLIFF (ACIDIC SUBTYPE) Concept: Piedmont Cliffs are steep to vertical, sparsely vegetated rock outcrops on river bluffs, lower slopes, and other topographically sheltered locations. The vegetation often includes some woody plants rooted in deeper soil pockets or crevices, but the overall woody cover is low. The Acidic Subtype encompasses the more common examples occurring on felsic igneous and metamorphic rocks and other acidic rocks and lacking flora indicative of base-rich conditions. Distinguishing Features: Piedmont Cliffs are distinguished from forest and shrubland communities by having contiguous rock outcroppings large enough to form a canopy break. In general, the minimum size is about 10 meters in height. Smaller rock outcrops that are completely shaded by the adjacent forest and that have few or no herbs typical of rock outcrops should not be treated as occurrences. Piedmont Cliffs are distinguished from various Piedmont and Mountain Glades and Barrens communities by having lower vegetation cover, with much bare rock present, herbaceous cover persistently less than 25 percent, and woody plants restricted to more favorable microsites. Cliffs generally have substantial vertical or very steep surfaces. Flat ledges on cliff faces and related vegetation on flatter tops of outcrops are included in the cliff occurrence. Piedmont Cliffs are distinguished from Granitic Flatrocks, which also have substantial bare rock, by having more fractured rock rather than a smooth exfoliated surface, and by having larger plants rooted in deeper soil microsites rather than primarily in shallow soil mats. Characteristic herbs such as Diamorpha smallii and Minuartia uniflora are present in Granitic Flatrocks and not on Piedmont Cliffs. -

Njplantlist.Pdf
List of Endangered Plant Species and Plant Species of Concern June 2016 Scientific Name Common Name G Rank S Rank Federal Status State Status Other Status Abies balsamea Balsam Fir G5 S1 E LP, HL Acorus americanus American Sweetflag G5 S1? HL Actaea rubra var. rubra Red Baneberry G5T5 S2 HL Adlumia fungosa Climbing Fumitory G4 S2 HL Aeschynomene virginica Sensitive Joint-vetch G2 S1 LT E LP, HL Agalinis auriculata Ear-leaf False Foxglove G3 SX HL Agalinis fasciculata Pine Barren Foxglove G5 S3 HL Agalinis paupercula var. paupercula Small-flower False Foxglove G5T5 S2 HL Agastache nepetoides Yellow Giant-hyssop G5 S2 HL Agastache scrophulariifolia Purple Giant-hyssop G4 S2 HL Agrimonia microcarpa Small-fruit Grooveburr G5 S2 HL Agrostis geminata Ticklegrass G5 S1? HL Alisma triviale Large Water-plantain G5 S1 E LP, HL Alopecurus aequalis var. aequalis Short-awn Meadow-foxtail G5T5 S2 HL Alopecurus carolinianus Tufted Meadow-foxtail G5 S3 HL Amaranthus pumilus Seabeach Amaranth G2 S1 LT E LP, HL Amelanchier humilis Low Service-berry G5 S1S2 HL Amelanchier nantucketensis Nantucket Service-berry G3Q S1 HL Amelanchier sanguinea var. sanguinea Round-leaf Service-berry G5T5 S1.1 E LP, HL Amelanchier stolonifera Running Service-berry G5 S3 HL Amianthium muscitoxicum Fly Poison G4G5 S2 HL Ammannia latifolia Koehn's Toothcup G5 S1 E LP, HL Andromeda polifolia var. glaucophylla Bog Rosemary G5T5 S1 E LP, HL Andropogon glomeratus var. hirsutior Hairy Beardgrass G5T5 SH.1 HL Andropogon gyrans Elliott's Beardgrass G5 S2 HL Andropogon ternarius var. ternarius Silvery Beardgrass G5T5? S2 HL Anemone canadensis Canada Anemone G5 SX HL Anemone cylindrica Long-head Anemone G5 S1 E LP, HL Anemone virginiana var. -

Alabama Inventory List
Alabama Inventory List The Rare, Threatened, & Endangered Plants & Animals of Alabama Alabama Natural August 2015 Heritage Program® TABLE OF CONTENTS INTRODUCTION .................................................................................................................................... 1 CHANGES FROM ALNHP TRACKING LIST OF OCTOBER 2012 ............................................... 3 DEFINITION OF HERITAGE RANKS ................................................................................................ 6 DEFINITIONS OF FEDERAL & STATE LISTED SPECIES STATUS ........................................... 8 VERTEBRATES ...................................................................................................................................... 10 Birds....................................................................................................................................................................................... 10 Mammals ............................................................................................................................................................................... 15 Reptiles .................................................................................................................................................................................. 18 Lizards, Snakes, and Amphisbaenas .................................................................................................................................. 18 Turtles and Tortoises ........................................................................................................................................................ -

REGIONAL FORESTER SENSITIVE PLANT SPECIES for the EASTERN REGION List Updated on February 20, 2012
REGIONAL FORESTER SENSITIVE PLANT SPECIES for the EASTERN REGION List Updated on February 20, 2012 KEY: R = Species is designated as Regional Forester Sensitive Species + = Species is present within the proclamation boundaries but is not at risk and is not designated as Regional Forester Sensitive Species EX = Species extirpated from the National Forest or Tallgrass Prairie NATIONAL FOREST/TALLGRASS PRAIRIE CODES: SOUTHERN TIER NEW ENGLAND/EASTERN GREAT LAKES ME - Midewin MO - Monongahela HM - Huron-Manistee MT - Mark Twain AL - Allegheny HI - Hiawatha SH - Shawnee FL - Finger Lakes OT - Ottawa HO - Hoosier GM - Green Mountain CN - Chequamegon-Nicolet WN - Wayne WM - White Mountain SU - Superior CP - Chippewa SCIENTIFIC NAME COMMON NAME NRANK GRANK CRITERIA ME MT SH HO WN MO AL FL GM WM HM HI OT CN SU CP NON-VASCULAR PLANTS Anzia colpodes Black Foam Lichen NNR G3G5 GTN R Arctoparmelia centrifuga Arctoparmelia Lichen NNR G3G5 GTN R Arctoparmelia subcentrifuga Arctoparmelia Lichen NNR G4G5 RISK R Atrichum crispum Atrichium Moss NNR G4 RISK R Baeomyces (=Dibaeis) absoluta Pink Dot Lichen NNR G4 RISK R Bryoxiphium norvegicum Norway Bryoxiphium Moss N4? G5? RISK R Bucklandiella microcarpa (=Racomitrium heterostichum) Rachomitrium Moss NNR G5 RISK R Caloplaca parvula N1 G1 GTN R R R R Campylium stellatum Arctic Star Campylium Moss NNR G5 RISK R + + + Cephaloziella elachista NNR G4 RISK R Cetraria (=Ahtiana) aurescens NNR G3G5 GTN + R R R Cladonia wainioi Wain's Cup Lichen NNR GNR RISK R Dichelyma capillaceum Dichelyma Moss NNR G5 RISK R Frullania -
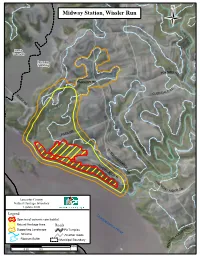
Midway Station, Wissler Run
Midway Station, Wissler Run F i s h i ng Cr e e k Martic Township Drumore Township NIS FUR S RD FU RN ISS RD D E R N DAL RIVER RD GLE n u r R ssle Wi PARK DR F E R N G L E S N U S D Q R U E H A N N O R C IV K E R D V R UE ek D re R C N ng Fishi H A R M O N R Y RI DGE D Lancaster County Natural Heritage Inventory Update 2008 Legend Susquehanna River Species of concern core habitat Natural Heritage Area Roads RD Supporting Landscape PA Turnpike W YORK O LL Streams All other roads O H PEACH BOTTOM N Riparian Buffer Municipal Boundary O T N E Miles B 0 0.25 0.5 1 Midway Station, Wissler Run Midway Station, Wissler Run – High significance PNHP Rank1 State Last Observed Species of Concern: Quality2 Global State Status1 (y-m-d) Plants: Bradley’s spleenwort (Asplenium bradleyi) G4 S1 PE 1995-10-30 BC Lobed spleenwort (Asplenium pinnatifidum) G4 S3 PR 1995-10-30 D American holly (Ilex opaca) G5 S2 PT 1995-10-30 D Cranefly orchid (Tipularia discolor) G4G5 S3 PR 2006-04-25 B 1 Please refer to Appendix IV for an explanation of PNHP ranks and legal status 2 Please refer to Appendix V for an explanation of quality ranks Location: o Municipalities: o Drumore township o USGS Quadrangles: o Holtwood Quadrangle o 1990 Lancaster County Natural Areas Inventory reference: o “Susquehannock State Park: SP564” – (Holtwood Quadrangle) Species of Concern Considerations: Plants: o Bradley’s spleenwort is a fern of very limited distribution in Pennsylvania. -

Endangered, Threatened, Watch List and Extirpated Plants of Indiana
Page 1 of 19 05/14/2021 Endangered, Threatened, Watch List and Extirpated Plants of Indiana Species Name Common Name GRANK SRANK FEDERAL STATE Acalypha deamii Deam's two-seeded mercury G4? S4 WL Aconitum uncinatum blue monkshood G4 S1 SE Acorus americanus sweetflag G5 S3? WL Actaea racemosa black bugbane G3G4 S4 WL Actaea rubifolia Appalachian bugbane G3 S1 SE Actaea rubra ssp. rubra red baneberry G5T5 S1? ST Adlumia fungosa climbing fumatory G4 SX SX Aesculus octandra yellow buckeye G5 S3? WL Agalinis auriculata earleaf foxglove G3 S2 ST Agalinis fasciculata clustered foxglove G5 S4S5 WL Agalinis gattingeri roundstem foxglove G4 S3 ST Agalinis skinneriana pale false foxglove G3G4 S2 ST Agrostis scabra rough bentgrass G5 S3? WL Alnus incana ssp. rugosa speckled alder G5T5 S3? WL Amelanchier humilis running serviceberry G5 S1 SE Ammophila breviligulata Marram grass G5 S2 WL Anaphalis margaritacea pearly everlasting G5 SX SX Andersonglossum boreale northern wild comfrey G5T4T5 SX SX Andromeda glaucophylla bog rosemary G5T5 S2 ST Andropogon ternarius silver bluestem G5 S4? WL Androsace occidentalis western rockjasmine G5 S2 ST Anemone caroliniana Carolina anemone G5 SX SX Antennaria solitaria single-head pussytoes G5 S4? WL Anticlea elegans var. glaucus white camas G5T4T5 S3 ST Arabis patens spreading rockcress G3 S1 SE Aralia hispida bristly sarsaparilla G5 S1 SE Arctostaphylos uva-ursi bearberry G5 S3 ST Arethusa bulbosa swamp-pink G5 SX SX Aristida longespica var. geniculata slim-spike three-awn grass G5T5? S3 WL Aristida tuberculosa seabeach -

Oklahoma Native Plant Record, Volume 7, Number 1, December 2007
61 Oklahoma Native Plant Record Volume 8, Number 1, December 2008 Fern Habitats and Rare Ferns in Oklahoma Dr. Bruce A. Smith McLoud High School McLoud, Oklahoma E-mail: [email protected] This paper features some of the more common fern habitats in Oklahoma and provides information on four rare Oklahoma ferns from two fern families: Aspleniaceae and Pteridaceae. Surprisingly, ferns can be found in a variety of habitats across Oklahoma. INTRODUCTION FERN HABITATS With over 2500 species of vascular plants One of the best places to look for ferns is (Taylor and Taylor 1991), Oklahoma is rich in on rock outcrops with mosses. Rocks are great both plant and habitat diversity. The vast places to find ferns, no matter what part of the majority of Oklahoma’s vascular plants are state you are in. Ferns can even be found flowering plants. Less than 100 species are ferns embedded in mosses on trees. If you can’t find and fern allies. Needless to say, ferns and fern them on rocks and trees, look for them in allies do not get the same attention as do marshes, bogs, mudflats, woodland forests, flowering plants. One obvious reason is that areas with rocky soils, near waterfalls, and even they are not as showy and do not catch our eye floating on the water surface. The places you as wildflowers do. Secondly, we tend to visit will likely not find them are in lawns or prairies. wildflower habitats more often than fern Often, when someone has brought or described habitats. Ferns live in some of the most to me the leaf of a “fern” they found in such a interesting places, however. -

Insecticidal Proteins and Methods for Their Use Insektizidproteine Und Verfahren Zu Deren Verwendung Protéines Insecticides Et Leurs Procédés D’Utilisation
(19) *EP003102684B1* (11) EP 3 102 684 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/82 (2006.01) C07K 14/415 (2006.01) 06.05.2020 Bulletin 2020/19 (86) International application number: (21) Application number: 15708059.9 PCT/US2015/014816 (22) Date of filing: 06.02.2015 (87) International publication number: WO 2015/120270 (13.08.2015 Gazette 2015/32) (54) INSECTICIDAL PROTEINS AND METHODS FOR THEIR USE INSEKTIZIDPROTEINE UND VERFAHREN ZU DEREN VERWENDUNG PROTÉINES INSECTICIDES ET LEURS PROCÉDÉS D’UTILISATION (84) Designated Contracting States: (74) Representative: J A Kemp LLP AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 14 South Square GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Gray’s Inn PL PT RO RS SE SI SK SM TR London WC1R 5JJ (GB) (30) Priority: 07.02.2014 US 201461937288 P (56) References cited: WO-A2-2013/098858 (43) Date of publication of application: 14.12.2016 Bulletin 2016/50 • H. K. ABICHT ET AL: "Genome Sequence of Desulfosporosinus sp. OT, an Acidophilic (73) Proprietors: Sulfate-Reducing Bacterium from Copper Mining • Pioneer Hi-Bred International, Inc. Waste in Norilsk, Northern Siberia", JOURNAL Johnston, Iowa 50131-1014 (US) OF BACTERIOLOGY, vol. 193, no. 21, 1 November • E. I. du Pont de Nemours and Company 2011 (2011-11-01), pages 6104-6105, Wilmington, DE 19805 (US) XP055196331, ISSN: 0021-9193, DOI: 10.1128/JB.06018-11 -& DATABASE UniProt (72) Inventors: [Online] 16 November 2011 (2011-11-16), • BARRY, Jennifer "SubName: Full=Uncharacterized protein Ames, Iowa 50014 (US) {ECO:0000313|EMBL:EGW36042.1};", • HAYES, Kevin XP002741068, retrieved from EBI accession no. -

W O 2015/120270 a L 1 3 August 2015 (13.08.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2015/120270 A l 1 3 August 2015 (13.08.2015) P O P C T (51) International Patent Classification: Drive, Urbandale, Iowa 50323 (US). LIU, Lu; 2303 Mid- C12N 15/82 (2006.01) C07K 14/415 (2006.01) dlefield Road, Palo Alto, California 94301 (US). SCHEP- ERS, Eric; 99 Water Wheel Drive, Port Deposit, Maryland (21) International Application Number: 21904 (US). YALPANI, Nasser; 6041 North Winwood PCT/US2015/014816 Drive, Johnston, Iowa 5013 1 (US). (22) International Filing Date: (74) Agent: BAUER, S . Christopher; E . I . du Pont d e 6 February 2015 (06.02.2015) Nemours and Company, Legal Patent Records Center, (25) Filing Language: English Chestnut Run Plaza 721/2640, 974 Centre Road, P O Box 2915 Wilmington, Delaware 19805 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 61/937,288 7 February 2014 (07.02.2014) U S AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (71) Applicants: PIONEER H I BRED INTERNATIONAL, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, INC. [US/US]; 7100 N.W. 62nd Avenue, Johnston, Iowa DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 5013 1-1014 (US). E . I . D U PONT D E NEMOURS AND HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, COMPANY [US/US]; 1007 Market Street, Wilmington, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, Delaware 19898 (US). -

We Have the Receipt of Fern-Seed, We Walk Invisible
We have the receipt of fern-seed, we walk invisible. Henry IV, Shakespeare Natural History of Ferns For many centuries in modern human history, naturalists have puzzled over the reproductive biology of ferns. Even though the fern lineage is among the oldest for vascular land plants, naturalists such as Linneaus tried desperately to find the missing seed of ferns. To Shakespeare and other Elizabethans, the seed of the fern was unknown and to have the recipe for the fern seed was to walk under the cloak of invisibility. The dust-like powder released from fern leaves was equated to pollen of flowering plants. Where pollen is the sperm transportation vehicle of flowering plants, the fern dust was the precursor for an alternate, alien, generation. My prior employment in the nursery business led to the realization that most modern humans have no insight to fern natural history. We expect homeowners to bring in their fern fronds once those regularly place spots on the leaves appeared. Most believed they were evidence of insect pest and none expected that they were just part of the life cycle. Ferns preceded dinosaurs in the history of the Earth. The ancestors of ferns began their intial diversification on land some 300 million years ago. While dinosaurs began their domination of the animal world 200 million years ago, it was ferns that reigned for land plants. It is estimated that at least half of the terrestrial forest of dinosaurian times was dominated by tree sized ferns, horsetails, and clubmosses. The sunlight captured by these plants over millions of years was used to generate energy-rich organic molecules as with modern plants.