Postcopulatory Sexual Selection in the Soldier Fly Merosargus Cingulatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TERRESTRIAL ARTHROPODS 2012-2016 BIOBLITZ VASHON ISLAND List Compiled By: Harsi Parker
COMPLETE LIST OF TERRESTRIAL ARTHROPODS 2012-2016 BIOBLITZ VASHON ISLAND List compiled by: Harsi Parker Number Species name Common name Notes Year Location Taxonomic Order 1 Gammaridae sp. scud 2016 J Amphipoda – Gammaridae 2 Hyalella sp. amphipod 2014, 2016 CH, J Amphipoda – Hyalellidae 3 Acari sp. #1 mite 2012, 2013, 2015, 2016 NP, SH, M, J Arachnida 4 Acari sp. #2 mite 2014 CH Arachnida 5 Opiliones sp. harvestman 2013, 2015 SH, M Arachnida 6 Callobius sp. hacklemesh weaver 2012 NP Arachnida – Amaurobiidae 7 Araneidae sp. orb weaver 2016 J Arachnida – Araneidae 8 Araneus diadematus Cross Orbweaver 2012, 2014 NP, CH Arachnida – Araneidae 9 Clubiona sp. leafcurling sac spider 2012 NP Arachnida – Clubionidae 10 Linyphiinae sp. sheetweb spider tentative ID 2012 NP Arachnida – Linyphiidae 11 Neriene sp. sheetweb spider tentative ID 2014 CH Arachnida – Linyphiidae 12 Pardosa sp. thinlegged wolf spider 2012 NP Arachnida – Lycosidae 13 Philodromus dispar running crab spider 2012 NP Arachnida – Philodromidae 14 Tibellus sp. slender crab spider tentative ID 2014 CH Arachnida – Philodromidae 15 Eris militaris Bronze Jumper tentative ID 2014 CH Arachnida – Salticidae 16 Metaphidippus manni jumping spider tentative ID 2014, 2016 CH, J Arachnida – Salticidae 17 Salticidae sp. #1 jumping spider 2014 CH Arachnida – Salticidae 18 Salticidae sp. #2 jumping spider 2015 M Arachnida – Salticidae 19 Salticus scenicus Zebra Jumper 2013, 2014, 2015 SH, CH, M Arachnida – Salticidae 20 Metellina sp. long-jawed orb weaver 2012 NP Arachnida – Tetragnathidae 21 Tetragnatha sp. long-jawed orb weaver 2013 SH Arachnida – Tetragnathidae 22 Theridiidae sp. cobweb spider 2012 NP Arachnida – Theridiidae 23 Misumena vatia Goldenrod Crab Spider 2013, 2016 SH, J Arachnida – Thomisidae 24 Thomisidae sp. -

Vertical and Horizontal Trophic Networks in the Aroid-Infesting Insect Community of Los Tuxtlas Biosphere Reserve, Mexico
insects Article Vertical and Horizontal Trophic Networks in the Aroid-Infesting Insect Community of Los Tuxtlas Biosphere Reserve, Mexico Guadalupe Amancio 1 , Armando Aguirre-Jaimes 1, Vicente Hernández-Ortiz 1,* , Roger Guevara 2 and Mauricio Quesada 3,4 1 Red de Interacciones Multitróficas, Instituto de Ecología A.C., Xalapa, Veracruz 91073, Mexico 2 Red de Biologia Evolutiva, Instituto de Ecología A.C., Xalapa, Veracruz 91073, Mexico 3 Laboratorio Nacional de Análisis y Síntesis Ecológica, Escuela Nacional de Estudios Superiores Unidad Morelia, Universidad Nacional Autónoma de México, Morelia 58190 Michoacán, Mexico 4 Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México, Morelia 58190 Michoacán, Mexico * Correspondence: [email protected] Received: 20 June 2019; Accepted: 9 August 2019; Published: 15 August 2019 Abstract: Insect-aroid interaction studies have focused largely on pollination systems; however, few report trophic interactions with other herbivores. This study features the endophagous insect community in reproductive aroid structures of a tropical rainforest of Mexico, and the shifting that occurs along an altitudinal gradient and among different hosts. In three sites of the Los Tuxtlas Biosphere Reserve in Mexico, we surveyed eight aroid species over a yearly cycle. The insects found were reared in the laboratory, quantified and identified. Data were analyzed through species interaction networks. We recorded 34 endophagous species from 21 families belonging to four insect orders. The community was highly specialized at both network and species levels. Along the altitudinal gradient, there was a reduction in richness and a high turnover of species, while the assemblage among hosts was also highly specific, with different dominant species. -

Investigations Into Mating Disruption, Delayed Mating, and Multiple Mating in Oriental Beetle, Anomala Orientalis (Waterhouse) (Coleoptera: Scarabaeidae)
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-2005 Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae). Erik J. Wenninger University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Wenninger, Erik J., "Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae)." (2005). Doctoral Dissertations 1896 - February 2014. 5748. https://scholarworks.umass.edu/dissertations_1/5748 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALS (WATERHOUSE) (COLEOPTERA: SCARABAEIDAE) A Dissertation Presented by ERIK J. WENNINGER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY September 2005 Plant, Soil & Insect Sciences Entomology Division © Copyright by Erik J. Wenninger 2005 All Rights Reserved INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALIS (WATERHOUSE), COLEOPTERA: SCARABAEIDAE A Dissertation Presented by ERIK J. WENNINGER Approved as to style and content by: Anne Averill, Chair Zii Joseph Elkinton, Member S Buonaccorsi, Member Peter Veneman, Department Head Department of Plant, Soil & Insect Sciences ACKNOWLEDGMENTS I am grateful to Dr. Anne Averill for allowing me the freedom to seek my own path. -
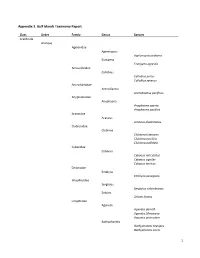
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

Acta Entomologica Slovenica, 29 (1), 2021 Zastopane Z Zanimivimi Najdbami, Kot So Suillia Gigantea (Meigen, 1830), S
ACTA ENTOMO LOGICA SL OVENICA LJUBLJANA, JUNIJ 2021 Vol. 29, øt. 1: 93 –106 Some familieS of Diptera from beer trapS in balaton HigHlanD, Hungary Libor Dvořák 1, kateřina Dvořáková 1, Jan Máca 2 & attila J. TráJer 3 1 Tři Sekery 21, cZ-35301 Mariánské Lázně, czech republic; e-mail: [email protected], [email protected] 2 Na Potoce 276, cZ-39181 veselí nad Lužnicí, czech republic; e-mail: [email protected] 3 Sustainability Solutions research Lab, University of Pannonia, egyetem utca 10, H-8200 veszprém, Hungary; e-mail: [email protected] abstract – Faunistic records for 41 Diptera species from nine families (anisopodidae, Drosophilidae, Dryomyzidae, Heleomyzidae, Lauxaniidae, Platystomatidae, Sciomyzi - dae, Syrphidae and Ulidiidae) collected at six sites at Felsőörs and Lovas in the Balaton Highland, Hungary are presented. amongst the material, the species Drosophila suzukii (Matsumura, 1931) (Drosophilidae) and Callopistromyia annulipes (Macquart, 1855) (Ulidiidae) belong to invasive pest species. Thermophilous species are represented by interesting records, namely Suillia gigantea (Meigen, 1830), S. lu - rida (Meigen, 1830) , S. variegata (Loew, 1862) (all Heleomyzidae), Minettia subvittata (Loew, 1847), Peplomyza discoidea (Meigen, 1830) (both Lauxaniidae), and Otites lamed (Schrank, 1781) (Ulidiidae). Furthermore, the disease vector role of Phortica variegata (Fallén, 1823) (Drosophilidae) is also discussed. key worDS : beer traps, Diptera, faunistics, Hungary izvleček – NekaJ DrUŽIN DvokrILcev IZ PIvSkIH PaSTI Na BaLa - ToNSkeM vIŠavJU Na MaDŽarSkeM Predstavljeni so favnistični podatki o 41 vrstah dvokrilcev iz devetih družin (anisopodidae, Drosophilidae, Dryomyzidae, Heleomyzidae, Lauxaniidae, Platys - tomatidae, Sciomyzidae, Syrphidae in Ulidiidae), zbranih na šestih krajih pri vaseh Felsőörs in Lovas na Balatonskem višavju na Madžarskem. -

Age-Related Sperm Production, Transfer, and Storage in The
Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae) Authors: Satoshi Hiroyoshi, Tsuguo Kohama, and Gadi V. P. Reddy The final publication is available at Springer via http://dx.doi.org/10.1007/s10905-016-9590-0. Hiroyoshi, Satoshi, Tsuguo Kohama, and Gadi V P Reddy. "Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae)." Journal of Insect Behavior (November 2016). DOI:https://dx.doi.org/10.1007/ s10905-016-9590-0. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Age-Related Sperm Production, Transfer, and Storage in the Sweet Potato Weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) Satoshi Hiroyoshi1,2 & Tsuguo Kohama 1,3 & Gadi V. P. Reddy4 1 Okinawa Prefectural Fruit-fly Eradication Program Office, 123 Maji, Naha, Okinawa 902-0072, Japan 2 7-12-203 Kotobukiso, Nishikawacho, Itoman, Okinawa 901-0304, Japan 3 Okinawa Prefectural Agricultural Research Center, 820 Makabe, Itoman, Okinawa 901-0336, Japan 4 Western Triangle Ag Research Center, Montana State University, 9546 Old Shelby Rd., P. O. Box 656, Conrad, MT 59425, USA Abstract The relationship between sperm production, insemination rate, and sperm transfer were studied in the sweet potato weevil, Cylas formicarius. Older adult males retained more sperm in the testes-seminal vesicle complex (TSC) and thus more was ejaculated into females at first mating. Number of matings per day for males was relatively constant across different ages, and frequent mating resulted in a reduced amount of sperm transferred to females, especially in young males. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION . WASHINGTON, D.C. Volume 121 1967 Number 3569 SOLDIER FLY LARVAE IN AMERICA NORTH OF MEXICO ' By Max W. McFadden ^ The Stratiomyidae or soldier flies are represented in America north of Mexico by approximately 237 species distributed through 37 genera. Prior to this study, larvae have been described for only 21 species representmg 15 genera. In addition to the lack of adequate descriptions and keys, classification has seldom been attempted and a phylogenetic treatment of the larvae has never been presented. The present study has been undertaken with several goals in mind: to rear and describe (1) as many species as possible; (2) to redescribe all previously described larvae of North American species; and (3), on the basis of larval characters, to attempt to define various taxo- nomic units and show phylogenetic relationships withm the family and between it and other closely related familes. Any attempt to establish subfamilial and generic lunits must be regarded as tentative. This is especially true in the present study since larvae of so many species of Stratiomyidae remain unknown. Undoubtably, as more species are reared, changes mil have to be made in keys and definitions of taxa. The keys have been prepared chiefly for identification of last mstar larvae. If earher mstars are known, they either have been 1 Modified from a Ph. D. dissertation submitted to the University of Alberta E(hnonton, Canada. ' 2 Entomology Research Division, U.S. Dept. Agriculture, Tobacco Insects Investigations, P.O. Box 1011, Oxford, N.C. 27565. : 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. -

Diptera, Stratiomyidae, Sarginae) Larvae
CORE Metadata, citation and similar papers at core.ac.uk Provided by MUCC (Crossref) Hindawi Publishing Corporation Psyche Volume 2012, Article ID 690203, 10 pages doi:10.1155/2012/690203 Research Article Use of Plant Resources by Merosargus (Diptera, Stratiomyidae, Sarginae) Larvae JulioC.R.Fontenelle,1 Flavia´ E. C. Viana-Silva,2 and Rogerio´ P. Martins3 1 Laboratorio´ de Pesquisas Ambientais, Coordenadoria de Meio Ambiente, Instituto Federal Minas Gerais (IFMG), Ouro Preto, MG 35400-000, Brazil 2 Conservac¸ao˜ e Manejo da Vida Silvestre, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 3 Departamento de Biologia, Universidade Federal do Ceara´ (UFC), Fortaleza, CE, Brazil Correspondence should be addressed to Julio C. R. Fontenelle, [email protected] Received 7 August 2012; Revised 26 September 2012; Accepted 26 September 2012 Academic Editor: Kleber Del-Claro Copyright © 2012 Julio C. R. Fontenelle et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The genus Merosargus (Loew) has 142 described species. This great diversity in the genus could be explained by larvae resource-use specialization. However, information on larval habitats is still very scarce. In Merosargus species, adult males defend oviposition sites, and this territorial behavior may lead to interspecific competition and make even more important the specialization and niche partitioning to prevent competitive exclusion. This study identified substrate types used as a resource by Merosargus larvae and investigated the degree of specialization and overlap in resource use by different species at an Atlantic forest remnant in Minas Gerais, Brazil. -

Ballyogan and Slieve Carran, Co. Clare
ISSN 1393 – 6670 N A T I O N A L P A R K S A N D W I L D L I F E S ERVICE IMPORTANT INVERTEBRATE AREA SURVEYS: BALLYOGAN AND SLIEVE CARRAN, CO. CLARE Adam Mantell & Roy Anderson I R I S H W ILDL I F E M ANUAL S 127 National Parks and Wildlife Service (NPWS) commissions a range of reports from external contractors to provide scientific evidence and advice to assist it in its duties. The Irish Wildlife Manuals series serves as a record of work carried out or commissioned by NPWS, and is one means by which it disseminates scientific information. Others include scientific publications in peer reviewed journals. The views and recommendations presented in this report are not necessarily those of NPWS and should, therefore, not be attributed to NPWS. Front cover, small photographs from top row: Limestone pavement, Bricklieve Mountains, Co. Sligo, Andy Bleasdale; Meadow Saffron Colchicum autumnale, Lorcan Scott; Garden Tiger Arctia caja, Brian Nelson; Fulmar Fulmarus glacialis, David Tierney; Common Newt Lissotriton vulgaris, Brian Nelson; Scots Pine Pinus sylvestris, Jenni Roche; Raised bog pool, Derrinea Bog, Co. Roscommon, Fernando Fernandez Valverde; Coastal heath, Howth Head, Co. Dublin, Maurice Eakin; A deep water fly trap anemone Phelliactis sp., Yvonne Leahy; Violet Crystalwort Riccia huebeneriana, Robert Thompson Main photograph: Burren Green Calamia tridens, Brian Nelson Important Invertebrate Area Surveys: Ballyogan and Slieve Carran, Co. Clare Adam Mantell1,2 and Roy Anderson3 1 42 Kernaghan Park, Annahilt, Hillsborough, Co. Down BT26 6DF, 2 Buglife Services Ltd., Peterborough, UK, 3 1 Belvoirview Park, Belfast BT8 7BL Keywords: Ireland, the Burren, insects, invertebrates, site inventory Citation: Mantell, A. -

Spatial and Temporal Variability of Necrophagous Diptera from Urban to Rural Areas
Medical and Veterinary Entomology (2005) 19, 379–391 Spatial and temporal variability of necrophagous Diptera from urban to rural areas C. HWANG1,3 andB. D. TURNER1,2 1Department of Life Sciences and 2Department of Forensic Science and Drug Monitoring, King’s College London, U.K. 3Department of Bioresources, Da-Yeh University, Da-Tsen, Chang-Hua County, Taiwan (current address). Abstract. The spatio-temporal variability of necrophagous fly assemblages in a linear series of habitats from central London to the rural surroundings in the south-west was studied using bottle traps between June 2001 and September 2002. A total of 3314 individuals in 20 dipteran families were identified from 127 sampling occasions. Calliphoridae accounted for 78.6% of all the dipteran speci- mens, with Calliphora vicina Robineau-Desvoidy, being the most abundant spe- cies (2603 individuals, 46.9%). Using canonical correspondence analyses (CCA) on 72 fly taxa, six sampled sites and 36 environmental variables, three habitat types corresponding to three groups of flies were identified. These were an urban habitat characterized by C. vicina, Lucilia illustris (Meigen) and L. sericata (Meigen), a rural grassland habitat, characterized by L. caesar (Linnaeus) and a rural woodland habitat characterized by Calliphora vomitoria (Linnaeus), Phaonia subventa (Harris), Neuroctena anilis (Falle´n) and Tephrochlamys flavipes (Zetterstedt). Intermediate species (L. ampullacea Villeneuve and P. pallida (Fabricius), located between the three habitats, were also found. Temporal abun- dance of the 10 most abundant species showed fluctuations between seasons, having low numbers of captured individuals during winter. Correspondence analysis showed clearly seasonal patterns at Box Hill site. The species–habitat associations suggest habitat differentiation between necrophagous guilds in this area and may be of ecological value. -

Dipterists Digest: Contents 1988–2021
Dipterists Digest: contents 1988–2021 Latest update at 12 August 2021. Includes contents for all volumes from Series 1 Volume 1 (1988) to Series 2 Volume 28(2) (2021). For more information go to the Dipterists Forum website where many volumes are available to download. Author/s Year Title Series Volume Family keyword/s EDITOR 2021 Corrections and changes to the Diptera Checklist (46) 2 28 (2): 252 LIAM CROWLEY 2021 Pandivirilia melaleuca (Loew) (Diptera, Therevidae) recorded from 2 28 (2): 250–251 Therevidae Wytham Woods, Oxfordshire ALASTAIR J. HOTCHKISS 2021 Phytomyza sedicola (Hering) (Diptera, Agromyzidae) new to Wales and 2 28 (2): 249–250 Agromyzidae a second British record Owen Lonsdale and Charles S. 2021 What makes a ‘good’ genus? Reconsideration of Chromatomyia Hardy 2 28 (2): 221–249 Agromyzidae Eiseman (Diptera, Agromyzidae) ROBERT J. WOLTON and BENJAMIN 2021 The impact of cattle on the Diptera and other insect fauna of a 2 28 (2): 201–220 FIELD temperate wet woodland BARRY P. WARRINGTON and ADAM 2021 The larval habits of Ophiomyia senecionina Hering (Diptera, 2 28 (2): 195–200 Agromyzidae PARKER Agromyzidae) on common ragwort (Jacobaea vulgaris) stems GRAHAM E. ROTHERAY 2021 The enigmatic head of the cyclorrhaphan larva (Diptera, Cyclorrhapha) 2 28 (2): 178–194 MALCOLM BLYTHE and RICHARD P. 2021 The biting midge Forcipomyia tenuis (Winnertz) (Diptera, 2 28 (2): 175–177 Ceratopogonidae LANE Ceratopogonidae) new to Britain IVAN PERRY 2021 Aphaniosoma melitense Ebejer (Diptera, Chyromyidae) in Essex and 2 28 (2): 173–174 Chyromyidae some recent records of A. socium Collin DAVE BRICE and RYAN MITCHELL 2021 Recent records of Minilimosina secundaria (Duda) (Diptera, 2 28 (2): 171–173 Sphaeroceridae Sphaeroceridae) from Berkshire IAIN MACGOWAN and IAN M. -

ISSUE 58, April, 2017
FLY TIMES ISSUE 58, April, 2017 Stephen D. Gaimari, editor Plant Pest Diagnostics Branch California Department of Food & Agriculture 3294 Meadowview Road Sacramento, California 95832, USA Tel: (916) 262-1131 FAX: (916) 262-1190 Email: [email protected] Welcome to the latest issue of Fly Times! As usual, I thank everyone for sending in such interesting articles. I hope you all enjoy reading it as much as I enjoyed putting it together. Please let me encourage all of you to consider contributing articles that may be of interest to the Diptera community for the next issue. Fly Times offers a great forum to report on your research activities and to make requests for taxa being studied, as well as to report interesting observations about flies, to discuss new and improved methods, to advertise opportunities for dipterists, to report on or announce meetings relevant to the community, etc., with all the associated digital images you wish to provide. This is also a great place to report on your interesting (and hopefully fruitful) collecting activities! Really anything fly-related is considered. And of course, thanks very much to Chris Borkent for again assembling the list of Diptera citations since the last Fly Times! The electronic version of the Fly Times continues to be hosted on the North American Dipterists Society website at http://www.nadsdiptera.org/News/FlyTimes/Flyhome.htm. For this issue, I want to again thank all the contributors for sending me such great articles! Feel free to share your opinions or provide ideas on how to improve the newsletter.