Age-Related Sperm Production, Transfer, and Storage in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Sweet Potato Weevil, Cylas Formicarius: a World Review
® Fruit, Vegetable and Cereal Science and Biotechnology ©2012 Global Science Books Management of Sweet Potato Weevil, Cylas formicarius: A World Review Rajasekhara Rao Korada1* • Samir Kanti Naskar2 • Archana Mukherjee1 • Cheruvandasseri Arumughan Jayaprakas2 1 Regional Centre of Central Tuber Crops Research Institute, Bhubaneswar - 751 019, India 2 Central Tuber Crops Research Institute, Sreekariyam, Thiruvananthapuram - 695 017, India Corresponding author : * [email protected] ABSTRACT Sweet potato is infested by many insect pests. Sweet potato weevil (SPW) Cylas formicarius (Fab.) is the important insect pest throughout the world, wherever it is grown. The weevil is managed by a package of practices together called integrated pest management (IPM). In India, a few genotypes of sweet potato have shown durable resistance throughout 2006 to 2011. A new screening method of germplasm, volatile-assisted selection (VAS), was developed to identify resistance/susceptibility in sweet potato genotypes based on the volatile chemicals that are released. Transgenic sweet potato was not successful at the field level. Farmers in Asia practice intercropping of sweet potato with ginger, bhendi, taro and yams to reduce the incidence of pests as well as to conserve soil moisture. Entomopathogenic fungi and nematodes are used successfully to control C. formicarius in the West and Latin America. Female sex pheromone (Z)-3-dodecen-1-ol (E)-2-butenoate has changed the pest dynamics in the field and has become an important tool in C. formicarius IPM. It was used to monitor and trap male weevils, thus reducing the reproductive success of female weevils. A number of botanical pesticides are available and their use is limited in developed countries. -

The Nestling Diet of Svalbard Snow Buntings Identified by DNA Metabarcoding
Faculty of Biosciences, Fisheries and Economics, Department of Arctic and Marine Biology The nestling diet of Svalbard snow buntings identified by DNA metabarcoding — Christian Stolz BIO-3950 Master thesis in Biology, Northern Populations and Ecosystems, May 2019 Faculty of Biosciences, Fisheries and Economics, Department of Arctic and Marine Biology The nestling diet of Svalbard snow buntings identified by DNA metabarcoding Christian Stolz, UiT The Arctic University of Norway, Tromsø, Norway and The University Centre in Svalbard (UNIS), Longyearbyen, Norway BIO-3950 Master Thesis in Biology, Northern Populations and Ecosystems, May 2018 Supervisors: Frode Fossøy, Norwegian Institute for Nature Research (NINA), Trondheim, Norway Øystein Varpe, The University Centre in Svalbard (UNIS), Longyearbyen, Norway Rolf Anker Ims, UiT The Arctic University of Norway, Tromsø, Norway i Abstract Tundra arthropods have considerable ecological importance as a food source for several bird species that are reproducing in the Arctic. The actual arthropod taxa comprising the chick diet are however rarely known, complicating assessments of ecological interactions. In this study, I identified the nestling diet of Svalbard snow bunting (Plectrophenax nivalis) for the first time. Faecal samples of snow bunting chicks were collected in Adventdalen, Svalbard in the breeding season 2018 and analysed via DNA metabarcoding. Simultaneously, the availability of prey arthropods was measured via pitfall trapping. The occurrence of 32 identified prey taxa in the nestling diet changed according to varying abundances and emergence patterns within the tun- dra arthropod community: Snow buntings provisioned their offspring mainly with the most abundant prey items which were in the early season different Chironomidae (Diptera) taxa and Scathophaga furcata (Diptera: Scathophagidae), followed by Spilogona dorsata (Diptera: Mus- cidae). -

Tropical Insect Chemical Ecology - Edi A
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol.VII - Tropical Insect Chemical Ecology - Edi A. Malo TROPICAL INSECT CHEMICAL ECOLOGY Edi A. Malo Departamento de Entomología Tropical, El Colegio de la Frontera Sur, Carretera Antiguo Aeropuerto Km. 2.5, Tapachula, Chiapas, C.P. 30700. México. Keywords: Insects, Semiochemicals, Pheromones, Kairomones, Monitoring, Mass Trapping, Mating Disrupting. Contents 1. Introduction 2. Semiochemicals 2.1. Use of Semiochemicals 3. Pheromones 3.1. Lepidoptera Pheromones 3.2. Coleoptera Pheromones 3.3. Diptera Pheromones 3.4. Pheromones of Insects of Medical Importance 4. Kairomones 4.1. Coleoptera Kairomones 4.2. Diptera Kairomones 5. Synthesis 6. Concluding Remarks Acknowledgments Glossary Bibliography Biographical Sketch Summary In this chapter we describe the current state of tropical insect chemical ecology in Latin America with the aim of stimulating the use of this important tool for future generations of technicians and professionals workers in insect pest management. Sex pheromones of tropical insectsUNESCO that have been identified to– date EOLSS are mainly used for detection and population monitoring. Another strategy termed mating disruption, has been used in the control of the tomato pinworm, Keiferia lycopersicella, and the Guatemalan potato moth, Tecia solanivora. Research into other semiochemicals such as kairomones in tropical insects SAMPLErevealed evidence of their presence CHAPTERS in coleopterans. However, additional studies are necessary in order to confirm these laboratory results. In fruit flies, the isolation of potential attractants (kairomone) from Spondias mombin for Anastrepha obliqua was reported recently. The use of semiochemicals to control insect pests is advantageous in that it is safe for humans and the environment. The extensive use of these kinds of technologies could be very important in reducing the use of pesticides with the consequent reduction in the level of contamination caused by these products around the world. -

Investigations Into Mating Disruption, Delayed Mating, and Multiple Mating in Oriental Beetle, Anomala Orientalis (Waterhouse) (Coleoptera: Scarabaeidae)
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-2005 Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae). Erik J. Wenninger University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Wenninger, Erik J., "Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae)." (2005). Doctoral Dissertations 1896 - February 2014. 5748. https://scholarworks.umass.edu/dissertations_1/5748 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALS (WATERHOUSE) (COLEOPTERA: SCARABAEIDAE) A Dissertation Presented by ERIK J. WENNINGER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY September 2005 Plant, Soil & Insect Sciences Entomology Division © Copyright by Erik J. Wenninger 2005 All Rights Reserved INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALIS (WATERHOUSE), COLEOPTERA: SCARABAEIDAE A Dissertation Presented by ERIK J. WENNINGER Approved as to style and content by: Anne Averill, Chair Zii Joseph Elkinton, Member S Buonaccorsi, Member Peter Veneman, Department Head Department of Plant, Soil & Insect Sciences ACKNOWLEDGMENTS I am grateful to Dr. Anne Averill for allowing me the freedom to seek my own path. -

EPPO Reporting Service
ORGANISATION EUROPEENNE EUROPEAN AND MEDITERRANEAN ET MEDITERRANEENNE PLANT PROTECTION POUR LA PROTECTION DES PLANTES ORGANIZATION EPPO Reporting Service NO. 02 PARIS, 2012-02-01 CONTENTS _______________________________________________________________________ Pests & Diseases 2012/023 - First report of Drosophila suzukii in Austria 2012/024 - Situation of Drosophila suzukii in Switzerland in 2011 2012/025 - Traps baited with a mixture of wine and vinegar are more attractive to Drosophila suzukii 2012/026 - Incursion of Ceratitis capitata in Austria 2012/027 - Incursion of Ceratitis capitata in Ile-de-France (FR) 2012/028 - First report of Tuta absoluta in Slovenia 2012/029 - First report of Tuta absoluta in Panama 2012/030 - Situation of Tuta absoluta in France in 2011 2012/031 - First report of Aproceros leucopoda in Slovenia 2012/032 - First report of Phyllocnistis vitegenella in Switzerland 2012/033 - First reports of Aphis illinoisensis in Cyprus, Italy, Libya, Malta, Montenegro and Spain 2012/034 - First reports of Grapevine flavescence dorée phytoplasma and its vector Scaphoideus titanus in Croatia 2012/035 - Maize redness: addition to the EPPO Alert List 2012/036 - First report of Potato spindle tuber viroid in Croatia 2012/037 - Pests newly found or intercepted in the Netherlands 2012/038 - New data on quarantine pests and pests of the EPPO Alert List 2012/039 - First report of Pomacea insularum (island apple snail) in Spain 2012/040 - Recent publications on forestry CONTENTS ____________________________________________________________________________ -

Repeatability of Sperm Size in Outbred and Inbred Scathophaga Stercoraria (Diptera: Scathophagidae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2007 Repeatability of sperm size in outbred and inbred Scathophaga stercoraria (Diptera: Scathophagidae) Bernasconi, G ; Ward, P I ; Hellriegel, B Abstract: Variability in male gametic traits can depend on several genetic and environmental factors such as developmental instability as a consequence of inbreeding, developmental noise during spermatogenesis, or age- or condition-dependent changes in allocation to sperm cells. Variation in sperm size is particularly evident in species that produce more than one sperm morph but also occurs among males in sperm- monomorphic species. Both discrete and continuous sperm size variation have been implicated in male fertilization success when the sperm of several males directly compete for fertilization of the same set of ova. In this study, we investigated among-male variation in sperm length in field-collected, outbred male Scathophaga stercoraria (L.) flies, as well as in flies from the same natural population that hadbeen subjected to 15 and 16 generations of inbreeding under laboratory conditions. Among-male variation in sperm length was significant and repeatable over subsequent matings in both inbred and outbred flies. We conclude that sperm length can be used as an individual male marker in sperm competition studies and that significant repeatability of sperm length supports heritability for this trait DOI: https://doi.org/10.4039/n06-063 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-154393 Journal Article Published Version Originally published at: Bernasconi, G; Ward, P I; Hellriegel, B (2007). -
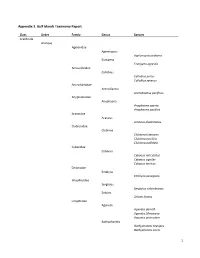
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -
Insect Morphology and Systematics (Ento-131) - Notes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/276175248 Insect Morphology and Systematics (Ento-131) - Notes Book · April 2010 CITATIONS READS 0 14,110 1 author: Cherukuri Sreenivasa Rao National Institute of Plant Health Management (NIPHM), Hyderabad, India 36 PUBLICATIONS 22 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Agricultural College, Jagtial View project ICAR-All India Network Project on Pesticide Residues View project All content following this page was uploaded by Cherukuri Sreenivasa Rao on 12 May 2015. The user has requested enhancement of the downloaded file. Insect Morphology and Systematics ENTO-131 (2+1) Revised Syllabus Dr. Cherukuri Sreenivasa Rao Associate Professor & Head, Department of Entomology, Agricultural College, JAGTIAL EntoEnto----131131131131 Insect Morphology & Systematics Prepared by Dr. Cherukuri Sreenivasa Rao M.Sc.(Ag.), Ph.D.(IARI) Associate Professor & Head Department of Entomology Agricultural College Jagtial-505529 Karminagar District 1 Page 2010 Insect Morphology and Systematics ENTO-131 (2+1) Revised Syllabus Dr. Cherukuri Sreenivasa Rao Associate Professor & Head, Department of Entomology, Agricultural College, JAGTIAL ENTO 131 INSECT MORPHOLOGY AND SYSTEMATICS Total Number of Theory Classes : 32 (32 Hours) Total Number of Practical Classes : 16 (40 Hours) Plan of course outline: Course Number : ENTO-131 Course Title : Insect Morphology and Systematics Credit Hours : 3(2+1) (Theory+Practicals) Course In-Charge : Dr. Cherukuri Sreenivasa Rao Associate Professor & Head Department of Entomology Agricultural College, JAGTIAL-505529 Karimanagar District, Andhra Pradesh Academic level of learners at entry : 10+2 Standard (Intermediate Level) Academic Calendar in which course offered : I Year B.Sc.(Ag.), I Semester Course Objectives: Theory: By the end of the course, the students will be able to understand the morphology of the insects, and taxonomic characters of important insects. -

Calyptratae: Diptera)
BUILDING THE TREE OF LIFE: RECONSTRUCTING THE EVOLUTION OF A RECENT AND MEGADIVERSE BRANCH (CALYPTRATAE: DIPTERA) SUJATHA NARAYANAN KUTTY (B.Tech) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF BIOLOGICAL SCIENCES NATIONAL UNIVERSITY OF SINGAPORE 2008 The great tragedy of Science - the slaying of a beautiful hypothesis by an ugly fact. - Thomas H. Huxley ii ACKNOWLEDGEMENTS We don't accomplish anything in this world alone... and whatever happens is the result of the whole tapestry of one's life and all the weavings of individual threads from one to another that creates something - Sandra Day O'Connor. The completion of this project would have been impossible without help from so many different quarters and the few lines of gratitude and acknowledgements written out in this section would do no justice to the actual amount of support and encouragement that I have received and that has contributed to making this study a successful endeavor. I am indebted to Prof. Meier for motivating me to embark on my PhD (at a very confusing point for me) and giving me a chance to explore a field that was quite novel to me. I express my sincere gratitude to him for all the guidance, timely advice, pep talks, and support through all the stages of this project and for always being patient while dealing with my ignorance. He has also been very understanding during all my non- academic distractions in the last two years. Thanks Prof.- your motivation and inspiration in the five years of my graduate study has given me the confidence to push the boundaries of my own capabilities. -

A Biological Switching Valve Evolved in the Female of a Sex-Role Reversed
RESEARCH ARTICLE A biological switching valve evolved in the female of a sex-role reversed cave insect to receive multiple seminal packages Kazunori Yoshizawa1*, Yoshitaka Kamimura2, Charles Lienhard3, Rodrigo L Ferreira4, Alexander Blanke5,6 1Laboratory of Systematic Entomology, School of Agriculture, Hokkaido University, Sapporo, Japan; 2Department of Biology, Keio University, Yokohama, Japan; 3Natural History Museum of Geneva, Geneva, Switzerland; 4Biology Department, Federal University of Lavras, Lavras, Brazil; 5Institute for Zoology, University of Cologne, Zu¨ lpicher, Ko¨ ln; 6Medical and Biological Engineering Research Group, School of Engineering and Computer Science, University of Hull, Hull, United Kingdom Abstract We report a functional switching valve within the female genitalia of the Brazilian cave insect Neotrogla. The valve complex is composed of two plate-like sclerites, a closure element, and in-and-outflow canals. Females have a penis-like intromittent organ to coercively anchor males and obtain voluminous semen. The semen is packed in a capsule, whose formation is initiated by seminal injection. It is not only used for fertilization but also consumed by the female as nutrition. The valve complex has two slots for insemination so that Neotrogla can continue mating while the first slot is occupied. In conjunction with the female penis, this switching valve is a morphological novelty enabling females to compete for seminal gifts in their nutrient-poor cave habitats through long copulation times and multiple seminal injections. The evolution of this switching valve may *For correspondence: have been a prerequisite for the reversal of the intromittent organ in Neotrogla. [email protected] DOI: https://doi.org/10.7554/eLife.39563.001 Competing interests: The authors declare that no competing interests exist. -

4.2.2 Sweetpotato Weevil, Cylas Brunneus (Fabricius) Synonyms
4.2.2 Sweetpotato weevil, Cylas brunneus (Fabricius) Synonyms: Cylas angustatus (Labram and Imhoff 1838; Labrouise 1842; Pierce 1940; Marshall 1953) Brentus brunneus (Olivier 1790) Taxonomic position: Insecta, Coleoptera, Brentidae Authors: P. Musana, J.S. Okonya, N. Mujica, P. Carhuapoma, & J. Kroschel Common name Sweetpotato weevil (English), Liberian sweetpotato borer (English), Charançon de la patate douce (French) Hosts Sweetpotato (Ipomoea batatas (L.) Lam), Morning glory (I. I. eriocarpa R. Br.), Water spinach (I. aquatica Forssk) Detection and identification Adults and larvae of Cylas brunneus damage both sweetpotato foliage and storage roots through their feeding activity. Adults mainly feed on the external surface of roots, causing round feeding punctures; they also feed on leaves, which causes irregular to round punctures. In stems, larvae burrow and feed, causing malformation, thickening, and cracking of the affected vines that reduce size and number of storage roots. The first signs of C. brunneus injury are mostly observed at or near the crown of roots exposed to the surface. Larvae feed inside the root and deposit frass in the feeding galleries or tunnels, thus reducing root quality and yield (Photo 1A, B). The feeding activity of the larvae induces the production of bitter terpenoid substances in the infested roots and renders them unfit for human or animal consumption. A B Photo 1. Sweetpotato root damage by sweetpotato weevil, Cylas brunneus: (A) adults and (B) larvae. Photos: Courtesy of CIP. Morphology Egg Eggs are oval, light yellow, and measure 0.7 X 0.5 mm (Photo 2A). Newly laid eggs are translucent and soft with rugose surface.