BMP Signaling Inhibition in Drosophila Secondary Cells Remodels the Seminal Proteome and Self and Rival Ejaculate Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Infection on the Secretory Capacity of the Male Accessory Glands
Clinical�������������� Urolo�y Infection and Secretory Capacity of Male Accessory Glands International Braz J Urol Vol. 35 (3): 299-309, May - June, 2009 Impact of Infection on the Secretory Capacity of the Male Accessory Glands M. Marconi, A. Pilatz, F. Wagenlehner, T. Diemer, W. Weidner Department of Urology and Pediatric Urology, University of Giessen, Giessen, Germany ABSTRACT Introduction: Studies that compare the impact of different infectious entities of the male reproductive tract (MRT) on the male accessory gland function are controversial. Materials and Methods: Semen analyses of 71 patients with proven infections of the MRT were compared with the results of 40 healthy non-infected volunteers. Patients were divided into 3 groups according to their diagnosis: chronic prostatitis NIH type II (n = 38), chronic epididymitis (n = 12), and chronic urethritis (n = 21). Results: The bacteriological analysis revealed 9 different types of microorganisms, considered to be the etiological agents, isolated in different secretions, including: urine, expressed prostatic secretions, semen and urethral smears: E. Coli (n = 20), Klebsiella (n = 2), Proteus spp. (n = 1), Enterococcus (n = 20), Staphylococcus spp. (n = 1), M. tuberculosis (n = 2), N. gonorrhea (n = 8), Chlamydia tr. (n = 16) and, Ureaplasma urealyticum (n = 1). The infection group had significantly (p < 0.05) lower: semen volume, alpha-glucosidase, fructose, and zinc in seminal plasma and, higher pH than the control group. None of these parameters was sufficiently accurate in the ROC analysis to discriminate between infected and non- infected men. Conclusion: Proven bacterial infections of the MRT impact negatively on all the accessory gland function parameters evaluated in semen, suggesting impairment of the secretory capacity of the epididymis, seminal vesicles and prostate. -

Morphology of the Male Reproductive Tract in the Water Scavenger Beetle Tropisternus Collaris Fabricius, 1775 (Coleoptera: Hydrophilidae)
Revista Brasileira de Entomologia 65(2):e20210012, 2021 Morphology of the male reproductive tract in the water scavenger beetle Tropisternus collaris Fabricius, 1775 (Coleoptera: Hydrophilidae) Vinícius Albano Araújo1* , Igor Luiz Araújo Munhoz2, José Eduardo Serrão3 1Universidade Federal do Rio de Janeiro, Instituto de Biodiversidade e Sustentabilidade (NUPEM), Macaé, RJ, Brasil. 2Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. 3Universidade Federal de Viçosa, Departamento de Biologia Geral, Viçosa, MG, Brasil. ARTICLE INFO ABSTRACT Article history: Members of the Hydrophilidae, one of the largest families of aquatic insects, are potential models for the Received 07 February 2021 biomonitoring of freshwater habitats and global climate change. In this study, we describe the morphology of Accepted 19 April 2021 the male reproductive tract in the water scavenger beetle Tropisternus collaris. The reproductive tract in sexually Available online 12 May 2021 mature males comprised a pair of testes, each with at least 30 follicles, vasa efferentia, vasa deferentia, seminal Associate Editor: Marcela Monné vesicles, two pairs of accessory glands (a bean-shaped pair and a tubular pair with a forked end), and an ejaculatory duct. Characters such as the number of testicular follicles and accessory glands, as well as their shape, origin, and type of secretion, differ between Coleoptera taxa and have potential to help elucidate reproductive strategies and Keywords: the evolutionary history of the group. Accessory glands Hydrophilid Polyphaga Reproductive system Introduction Coleoptera is the most diverse group of insects in the current fauna, The evolutionary history of Coleoptera diversity (Lawrence et al., with about 400,000 described species and still thousands of new species 1995; Lawrence, 2016) has been grounded in phylogenies with waiting to be discovered (Slipinski et al., 2011; Kundrata et al., 2019). -

Investigations Into Mating Disruption, Delayed Mating, and Multiple Mating in Oriental Beetle, Anomala Orientalis (Waterhouse) (Coleoptera: Scarabaeidae)
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-2005 Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae). Erik J. Wenninger University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Wenninger, Erik J., "Investigations into mating disruption, delayed mating, and multiple mating in oriental beetle, Anomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae)." (2005). Doctoral Dissertations 1896 - February 2014. 5748. https://scholarworks.umass.edu/dissertations_1/5748 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALS (WATERHOUSE) (COLEOPTERA: SCARABAEIDAE) A Dissertation Presented by ERIK J. WENNINGER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY September 2005 Plant, Soil & Insect Sciences Entomology Division © Copyright by Erik J. Wenninger 2005 All Rights Reserved INVESTIGATIONS INTO MATING DISRUPTION, DELAYED MATING, AND MULTIPLE MATING IN ORIENTAL BEETLE, ANOMALA ORIENTALIS (WATERHOUSE), COLEOPTERA: SCARABAEIDAE A Dissertation Presented by ERIK J. WENNINGER Approved as to style and content by: Anne Averill, Chair Zii Joseph Elkinton, Member S Buonaccorsi, Member Peter Veneman, Department Head Department of Plant, Soil & Insect Sciences ACKNOWLEDGMENTS I am grateful to Dr. Anne Averill for allowing me the freedom to seek my own path. -
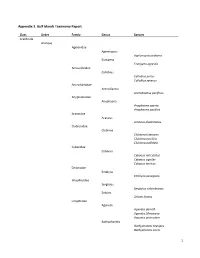
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

BMP Signaling Inhibition in Drosophila Secondary Cells Remodels the Seminal Proteome and Self and Rival Ejaculate Functions
BMP signaling inhibition in Drosophila secondary cells remodels the seminal proteome and self and rival ejaculate functions Ben R. Hopkinsa,1,2, Irem Sepila, Sarah Bonhamb, Thomas Millera, Philip D. Charlesb, Roman Fischerb, Benedikt M. Kesslerb, Clive Wilsonc, and Stuart Wigbya aEdward Grey Institute, Department of Zoology, University of Oxford, OX1 3PS Oxford, United Kingdom; bTarget Discovery Institute (TDI) Mass Spectrometry Laboratory, Target Discovery Institute, Nuffield Department of Medicine, University of Oxford, OX3 7BN Oxford, United Kingdom; and cDepartment of Physiology, Anatomy, and Genetics, University of Oxford, OX1 3QX Oxford, United Kingdom Edited by David L. Denlinger, The Ohio State University, Columbus, OH, and approved October 22, 2019 (received for review August 22, 2019) Seminal fluid proteins (SFPs) exert potent effects on male and sequestering SFPs in different cells or glands, males are afforded female fitness. Rapidly evolving and molecularly diverse, they control over their release and, consequently, afforded spatiotem- derive from multiple male secretory cells and tissues. In Drosophila poral control over their interactions with sperm, the female re- melanogaster, most SFPs are produced in the accessory glands, productive tract, and with other SFPs (16). Additionally, functional which are composed of ∼1,000 fertility-enhancing “main cells” diversification of tissues and cell types may be required to build and ∼40 more functionally cryptic “secondary cells.” Inhibition of specialized parts of the ejaculate, such as mating plugs (17). In bone morphogenetic protein (BMP) signaling in secondary cells either case, activities may be carried out independently between suppresses secretion, leading to a unique uncoupling of normal cell types and tissues or there may be cross-talk between them female postmating responses to the ejaculate: refractoriness stim- that coordinates global seminal fluid composition. -

Viewed in Turner and Inflammatory Disorders (Wong Et Al
INVESTIGATION Neprilysins: An Evolutionarily Conserved Family of Metalloproteases That Play Important Roles in Reproduction in Drosophila Jessica L. Sitnik,* Carmen Francis,†,‡,1 Korneel Hens,†,‡ Roger Huybrechts,§ Mariana F. Wolfner,*,3 and Patrick Callaerts†,‡,3 *Department of Molecular Biology and Genetics, Cornell University, Ithaca, New York 14853, †Laboratory of Behavioral and Developmental Genetics, KULeuven, 3000 Leuven, Belgium, ‡VIB Center for the Biology of Disease, 3000 Leuven, Belgium, and §Zoological Institute, KULeuven, 3000 Leuven, Belgium ABSTRACT Members of the M13 class of metalloproteases have been implicated in diseases and in reproductive fitness. Nevertheless, their physiological role remains poorly understood. To obtain a tractable model with which to analyze this protein family’s function, we characterized the gene family in Drosophila melanogaster and focused on reproductive phenotypes. The D. melanogaster genome contains 24 M13 class protease homologs, some of which are orthologs of human proteases, including neprilysin. Many are expressed in the reproductive tracts of either sex. Using RNAi we individually targeted the five Nep genes most closely related to vertebrate neprilysin, Nep1-5, to investigate their roles in reproduction. A reduction in Nep1, Nep2,orNep4 expression in females reduced egg laying. Nep1 and Nep2 are required in the CNS and the spermathecae for wild-type fecundity. Females that are null for Nep2 also show defects as hosts of sperm competition as well as an increased rate of depletion for stored sperm. Furthermore, eggs laid by Nep2 mutant females are fertilized normally, but arrest early in embryonic development. In the male, only Nep1 was required to induce normal patterns of female egg laying. -

Comparative Gross Morphology of Male Accessory Glands Among Neotropical Muridae (Mammalia: Rodentia) with Comments on Systematic Implications
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 159 Comparative Gross Morphology of Male Accessory Glands among Neotropical Muridae (Mammalia: Rodentia) with Comments on Systematic Implications by Robert S. Voss Div&.~n of Mammals Museum of Zoology University of Michigan Ann Arbor, Michigan 48109 and Alicia V. Linzey Department of Biology Virginia Polytechnic Institute and State University Blacksburg, Virginia 2406 1 Ann Arbor MUSEUM OF ZOOLOGY, UNlVERSI2'Y OF MICHIGAN May 20, 1981 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN WILLIAM D. HAMILTON, EDITOR The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mrs. Bradshaw, H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109. -

Binucleation of Male Accessory Gland Cells in the Common Bed Bug Cimex Lectularius
UC Riverside UC Riverside Previously Published Works Title Binucleation of male accessory gland cells in the common bed bug Cimex lectularius. Permalink https://escholarship.org/uc/item/705958ck Journal Scientific reports, 9(1) ISSN 2045-2322 Authors Takeda, Koji Yamauchi, Jun Miki, Aoi et al. Publication Date 2019-04-24 DOI 10.1038/s41598-019-42844-0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificreports OPEN Binucleation of male accessory gland cells in the common bed bug Cimex lectularius Received: 2 October 2018 Koji Takeda1, Jun Yamauchi1, Aoi Miki1, Daeyun Kim2,3, Xin-Yeng Leong 2,4, Accepted: 10 April 2019 Stephen L. Doggett5, Chow-Yang Lee2 & Takashi Adachi-Yamada1 Published: xx xx xxxx The insect male accessory gland (MAG) is an internal reproductive organ responsible for the synthesis and secretion of seminal fuid components, which play a pivotal role in the male reproductive strategy. In many species of insects, the efective ejaculation of the MAG products is essential for male reproduction. For this purpose, the fruit fy Drosophila has evolved binucleation in the MAG cells, which causes high plasticity of the glandular epithelium, leading to an increase in the volume of seminal fuid that is ejaculated. However, such a binucleation strategy has only been sporadically observed in Dipteran insects, including fruit fies. Here, we report the discovery of binucleation in the MAG of the common bed bug, Cimex lectularius, which belongs to hemimetabolous Hemiptera phylogenetically distant from holometabolous Diptera. In Cimex, the cell morphology and timing of synchrony during binucleation are quite diferent from those of Drosophila. -

Mrna Poly(A)-Tail Changes Specified by Deadenylation Broadly Reshape Translation in Drosophila Oocytes and Early Embryos
mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Eichhorn, Stephen W et al. “mRNA poly(A)-Tail Changes Specified by Deadenylation Broadly Reshape Translation in Drosophila Oocytes and Early Embryos.” eLife 5 (2016): n. pag. As Published http://dx.doi.org/10.7554/eLife.16955 Publisher eLife Sciences Publications, Ltd. Version Final published version Citable link http://hdl.handle.net/1721.1/106506 Terms of Use Creative Commons Attribution 4.0 International License Detailed Terms http://creativecommons.org/licenses/by/4.0/ RESEARCH ARTICLE mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos Stephen W Eichhorn1,2, Alexander O Subtelny1,2,3, Iva Kronja2,4, Jamie C Kwasnieski1,2, Terry L Orr-Weaver2,4*, David P Bartel1,2* 1Howard Hughes Medical Institute, Whitehead Institute for Biomedical Research, Cambridge, United States; 2Department of Biology, Massachusetts Institute of Technology, Cambridge, United States; 3Harvard-MIT Division of Health Sciences and Technology, Cambridge, United States; 4Whitehead Institute for Biomedical Research, Cambridge, United States Abstract Because maturing oocytes and early embryos lack appreciable transcription, posttranscriptional regulatory processes control their development. To better understand this control, we profiled translational efficiencies -

Age-Related Sperm Production, Transfer, and Storage in The
Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae) Authors: Satoshi Hiroyoshi, Tsuguo Kohama, and Gadi V. P. Reddy The final publication is available at Springer via http://dx.doi.org/10.1007/s10905-016-9590-0. Hiroyoshi, Satoshi, Tsuguo Kohama, and Gadi V P Reddy. "Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae)." Journal of Insect Behavior (November 2016). DOI:https://dx.doi.org/10.1007/ s10905-016-9590-0. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Age-Related Sperm Production, Transfer, and Storage in the Sweet Potato Weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) Satoshi Hiroyoshi1,2 & Tsuguo Kohama 1,3 & Gadi V. P. Reddy4 1 Okinawa Prefectural Fruit-fly Eradication Program Office, 123 Maji, Naha, Okinawa 902-0072, Japan 2 7-12-203 Kotobukiso, Nishikawacho, Itoman, Okinawa 901-0304, Japan 3 Okinawa Prefectural Agricultural Research Center, 820 Makabe, Itoman, Okinawa 901-0336, Japan 4 Western Triangle Ag Research Center, Montana State University, 9546 Old Shelby Rd., P. O. Box 656, Conrad, MT 59425, USA Abstract The relationship between sperm production, insemination rate, and sperm transfer were studied in the sweet potato weevil, Cylas formicarius. Older adult males retained more sperm in the testes-seminal vesicle complex (TSC) and thus more was ejaculated into females at first mating. Number of matings per day for males was relatively constant across different ages, and frequent mating resulted in a reduced amount of sperm transferred to females, especially in young males. -

Novel Seminal Fluid Proteins in the Seed Beetle Callosobruchus
Insect Molecular Biology (2017) 26(1), 58–73 doi: 10.1111/imb.12271 Novel seminal fluid proteins in the seed beetle Callosobruchus maculatus identified by a proteomic and transcriptomic approach H. Bayram, A. Sayadi, J. Goenaga*, E. Immonen and are SFPs. These results can inform further investiga- G. Arnqvist tion, to better understand the molecular mechanisms Department of Ecology and Genetics, Evolutionary of sexual conflict in seed beetles. Biology Centre, Uppsala University, Uppsala, Sweden Keywords: evolution, reproduction, coleoptera, seminal fluid. Abstract The seed beetle Callosobruchus maculatus is a sig- Introduction nificant agricultural pest and increasingly studied Ejaculates are a complex mixture of sperm and seminal model of sexual conflict. Males possess genital fluid (Perry et al., 2013). Seminal fluid proteins (SFPs) are spines that increase the transfer of seminal fluid pro- being increasingly studied owing to their effects on fertility teins (SFPs) into the female body. As SFPs alter and female physiology (Poiani, 2006). SFPs improve male female behaviour and physiology, they are likely to fertility directly, maintaining sperm viability and motility (eg modulate reproduction and sexual conflict in this King et al., 2011; Simmons & Beveridge, 2011; Smith & species. Here, we identified SFPs using proteomics Stanfield, 2012). Furthermore, many SFPs influence combined with a de novo transcriptome. A prior 2D- female traits, such as egg production and remating rate sodium dodecyl sulphate polyacrylamide gel electro- (Mane et al., 1983; Chapman, 2001) and are therefore likely phoresis analysis identified male accessory gland targets of selection via sexual conflict (Arnqvist & Rowe, protein spots that were probably transferred to the 2005; Sirot et al., 2014). -

Physiological Functions and Evolutionary Dynamics of Male Seminal Proteins in Drosophila
Heredity (2002) 88, 85–93 2002 Nature Publishing Group All rights reserved 0018-067X/02 $25.00 www.nature.com/hdy The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila MF Wolfner Department of Molecular Biology and Genetics, Cornell University, Ithaca NY 14853–2703, USA During mating, males transfer seminal proteins and pep- their inhibitors, and lipases. An apparent prohormonal Acp, tides, along with sperm, to their mates. In Drosophila mel- ovulin (Acp26Aa) stimulates ovulation in mated Drosophila anogaster, seminal proteins made in the male’s accessory females. Another male-derived protein, the large glyco- gland stimulate females’ egg production and ovulation, protein Acp36DE, is needed for sperm storage in the mated reduce their receptivity to mating, mediate sperm storage, female and through this action can also affect sperm pre- cause part of the survival cost of mating to females, and may cedence, indirectly. A third seminal protein, the protease protect reproductive tracts or gametes from microbial attack. inhibitor Acp62F, is a candidate for contributing to the sur- The physiological functions of these proteins indicate that vival cost of mating, given its toxicity in ectopic expression males provide their mates with molecules that initiate assays. That male-derived molecules manipulate females in important reproductive responses in females. A new com- these ways can result in a molecular conflict between the prehensive EST screen, in conjunction with earlier screens, sexes that can drive the rapid evolution of Acps. Supporting has identified ෂ90% of the predicted secreted accessory this hypothesis, an unusually high fraction of Acps show gland proteins (Acps).