The Loranthaceae of Madagascar and of the Neighboring Archipelagos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liste Candidatures Conseillers Alaotra Mangoro
NOMBRE DISTRICT COMMUNE ENTITE NOM ET PRENOM(S) CANDIDATS CANDIDATS AMBATONDRAZAKA AMBANDRIKA 1 RTM (Refondation Totale De Madagascar) RAKOTOZAFY Jean Marie Réné AMBATONDRAZAKA AMBANDRIKA 1 MMM (Malagasy Miara-Miainga) ARIMAHANDRIZOA Raherinantenaina INDEPENDANT RANAIVOARISON HERINJIVA AMBATONDRAZAKA AMBANDRIKA 1 RANAIVOARISON Herinjiva (Ranaivoarison Herinjiva) AMBATONDRAZAKA AMBANDRIKA 1 IRD (Isika Rehetra Miaraka @ Andry Rajoelina) RANDRIARISON Célestin AMBATONDRAZAKA AMBATONDRAZAKA 1 TIM (Tiako I Madagasikara) RANDRIAMANARINA - INDEPENDANT RAZAKAMAMONJY HAJASOA AMBATONDRAZAKA AMBATONDRAZAKA 1 RAZAKAMAMONJY Hajasoa Mazarin MAZARIN (Razakamamonjy Hajasoa Mazarin) INDEPENDANT RAHARIJAONA ROJO AMBATONDRAZAKA AMBATONDRAZAKA 1 RAHARIJAONA Rojo (Raharijaona Rojo) AMBATONDRAZAKA AMBATONDRAZAKA 1 IRD (Isika Rehetra Miaraka @ Andry Rajoelina) RATIANARIVO Jean Cyprien Roger AMBATONDRAZAKA AMBATONDRAZAKA 1 IRD (Isika Rehetra Miaraka @ Andry Rajoelina) RABEVASON Hajatiana Thierry Germain SUBURBAINE AMBATONDRAZAKA INDEPENDANT RANDRIANASOLO ROLLAND AMBATONDRAZAKA 1 RANDRIANASOLO Rolland SUBURBAINE (Randrianasolo Rolland) AMBATONDRAZAKA AMBATONDRAZAKA 1 MMM (Malagasy Miara-Miainga) RAKOTONDRASOA Emile SUBURBAINE AMBATONDRAZAKA AMBATONDRAZAKA 1 TIM (Tiako I Madagasikara) RANJAKASOA Albert SUBURBAINE INDEPENDANT RANDRIAMAHAZO FIDISOA AMBATONDRAZAKA AMBATOSORATRA 1 HERINIAINA (Randriamahazo Fidisoa RANDRIAMAHAZO Fidisoa Heriniaina Heriniaina) AMBATONDRAZAKA AMBATOSORATRA 1 IRD (Isika Rehetra Miaraka @ Andry Rajoelina) RANDRIANANTOANDRO Gérard AMBATONDRAZAKA -

Chapitre 3 Conditions Socio-Economiques Et Problematique De La Zone D’Etude
CHAPITRE 3 CONDITIONS SOCIO-ECONOMIQUES ET PROBLEMATIQUE DE LA ZONE D’ETUDE 3.1 Conditions socio-économiques actuelles 3.1.1 Système administratif, zone de démarcation et population La zone de l’étude, à savoir les 2 districts, 9 communes et 52 villages, comme indiqué dans le tableau suivant, est administrativement sous la juridiction de la région d’Alaotra-Mangoro. Géographiquemnt, la zone de l’étude comprend le bassin versant de la rivière Sahabe, lesbassin versant de la rivière Sahamilahy, les bassins de 4 petits et moyens cours d’eau, et la zone du PC 23. La délimitation administrative est illustrée à la Fig. 3.1. Tableau 3.1.1 Unités et zones administratives dans la zone de l’étude Nombre Région District Commune de Zone villages Ampasikely 4 4 petits et moyens bassins fluviaux Andrebakely 6 4 petits et moyens bassins Sud fluviaux 4 petits et moyens bassins Ambatomainty 9 Amparafaravola fluviaux, zone du PC 23 Bassin de la rivière Sahamilahy, Alaotra- Morarano bassin de la rivière Sahabe, 4 27 Mangoro Chrome petits et moyens bassins fluviaux, zone du PC23 Ranomanity 6 Bassin de la rivière Sahabe Bejofo 2 Bassin de la rivière Sahabe Soalazaina 5 Bassin de la rivière Sahabe Ambatondrazaka Tanambao 6 Bassin de la rivière Sahabe Besakay Andilanatoby 6 Bassin de la rivière Sahabe Source: Bureau regional d’Alaotra-Mangoro D’après une étude supplémentaire par le biais d’interviews menée en 2006, le total de la population dans tous les villages de la zone de l’étude est de 118.194 personnes, le nombre de foyers de 20.631, et la taille d’une famille moyenne de 5,7 personnes. -

Doctorat De L'université De Toulouse
En vue de l’obt ention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par : Université Toulouse 3 Paul Sabatier (UT3 Paul Sabatier) Discipline ou spécialité : Ecologie, Biodiversité et Evolution Présentée et soutenue par : Joeri STRIJK le : 12 / 02 / 2010 Titre : Species diversification and differentiation in the Madagascar and Indian Ocean Islands Biodiversity Hotspot JURY Jérôme CHAVE, Directeur de Recherches CNRS Toulouse Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Frédéric MEDAIL, Professeur à l'Université Paul Cezanne Aix-Marseille Christophe THEBAUD, Professeur à l'Université Paul Sabatier Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : UMR 5174 CNRS-UPS Evolution & Diversité Biologique Directeur(s) de Thèse : Christophe THEBAUD Rapporteurs : Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Contents. CONTENTS CHAPTER 1. General Introduction 2 PART I: ASTERACEAE CHAPTER 2. Multiple evolutionary radiations and phenotypic convergence in polyphyletic Indian Ocean Daisy Trees (Psiadia, Asteraceae) (in preparation for BMC Evolutionary Biology) 14 CHAPTER 3. Taxonomic rearrangements within Indian Ocean Daisy Trees (Psiadia, Asteraceae) and the resurrection of Frappieria (in preparation for Taxon) 34 PART II: MYRSINACEAE CHAPTER 4. Phylogenetics of the Mascarene endemic genus Badula relative to its Madagascan ally Oncostemum (Myrsinaceae) (accepted in Botanical Journal of the Linnean Society) 43 CHAPTER 5. Timing and tempo of evolutionary diversification in Myrsinaceae: Badula and Oncostemum in the Indian Ocean Island Biodiversity Hotspot (in preparation for BMC Evolutionary Biology) 54 PART III: MONIMIACEAE CHAPTER 6. Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long distance dispersal, but not West Gondwana (accepted in Journal of Biogeography) 72 CHAPTER 7 General Discussion 86 REFERENCES 91 i Contents. -

Epiparasitism in Phoradendron Durangense and P. Falcatum (Viscaceae) Clyde L
Aliso: A Journal of Systematic and Evolutionary Botany Volume 27 | Issue 1 Article 2 2009 Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae) Clyde L. Calvin Rancho Santa Ana Botanic Garden, Claremont, California Carol A. Wilson Rancho Santa Ana Botanic Garden, Claremont, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Calvin, Clyde L. and Wilson, Carol A. (2009) "Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 27: Iss. 1, Article 2. Available at: http://scholarship.claremont.edu/aliso/vol27/iss1/2 Aliso, 27, pp. 1–12 ’ 2009, Rancho Santa Ana Botanic Garden EPIPARASITISM IN PHORADENDRON DURANGENSE AND P. FALCATUM (VISCACEAE) CLYDE L. CALVIN1 AND CAROL A. WILSON1,2 1Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711-3157, USA 2Corresponding author ([email protected]) ABSTRACT Phoradendron, the largest mistletoe genus in the New World, extends from temperate North America to temperate South America. Most species are parasitic on terrestrial hosts, but a few occur only, or primarily, on other species of Phoradendron. We examined relationships among two obligate epiparasites, P. durangense and P. falcatum, and their parasitic hosts. Fruit and seed of both epiparasites were small compared to those of their parasitic hosts. Seed of epiparasites was established on parasitic-host stems, leaves, and inflorescences. Shoots developed from the plumular region or from buds on the holdfast or subjacent tissue. The developing endophytic system initially consisted of multiple separate strands that widened, merged, and often entirely displaced its parasitic host from the cambial cylinder. -

Epoa) Phone: +261 34 54 463 44; Email: Coordo [email protected]
Emergency Plan of Action Madagascar: Heavy Rains, Floods and Landslides DREF Operation n° MDRMG016 Glide n°: F-2020-0008-MDG Date of issue: 05/02/2020 Expected timeframe: 4 months Expected end date 30/06/2020 Category allocated to the of the disaster or crisis: Yellow DREF allocated: CHF 307,356 Total number of people affected: 106,846 Number of people to 5,000 (1,000 HHs) be assisted: Provinces affected: Alaotra Mangoro, Provinces/Regions Alaotra Mangoro, Analamanga, targeted: Analamanga, and Betsiboka, Boeny, Betsiboka, Diana, Melaky, Host National Society presence (n° of volunteers, staff, branches): 143 Red Cross Red Crescent Movement partners actively involved in the operation: French Red Cross (PIROI), German Red Cross, and Luxemburg Red Cross Other partner organizations actively involved in the operation: BNGRC (Bureau National de Gestion des Risques de Catastrophes) A. Situation analysis Description of the disaster On 17 January, the Weather Service published a Communique on the risk (low to moderate) of cyclogenesis in the Mozambique Channel, and on 19 January the cyclonic circulation called Zone de Convergence Inter-Tropicale (ZCIT) is fed by the monsoon flow on the North of the Channel to the Northwest of Madagascar. The related storm made landfall on 22nd January on the West coast of Madagascar, in the district of Besalampy, the Melaky region. Red Alert Warning for heavy rains was issued for the following regions on the 22nd January: Boeny, Sofia (Districts of Analalava, Antsohihy, Mampikomy, Boriziny, and Mandritsara), Yellow Alert Warning was issued for the region of Analamanga and Alaotra Mangoro, On 23rd January, Red Alert for High wind for the majority of the coast of the country, from the North, North-Eastern, North-Western, and Western regions. -

Répartition De La Caisse-École 2020 Des Collèges D'enseignement
Repartition de la caisse-école 2020 des Collèges d'Enseignement Général DREN ALAOTRA-MANGORO CISCO AMBATONDRAZAKA Prestataire OTIV ALMA Commune Code Etablissement Montant AMBANDRIKA 503010005 CEG AMBANDRIKA 1 598 669 AMBATONDRAZAKA 503020018 C.E.G. ANOSINDRAFILO 1 427 133 AMBATONDRAZAKA 503020016 CEG RAZAKA 3 779 515 AMBATONDRAZAKA SUBURBAINE 503030002 C.E.G. ANDINGADINGANA 1 142 422 AMBATOSORATRA 503040001 CEG AMBATOSORATRA 1 372 802 AMBOHIBOROMANGA 503070012 CEG ANNEXE AMBOHIBOROMANGA 878 417 AMBOHIBOROMANGA 503150018 CEG ANNEXE MARIANINA 775 871 AMBOHIBOROMANGA 503150016 CEGFERAMANGA SUD 710 931 AMBOHIDAVA 503040017 CEG AMBOHIDAVA 1 203 171 AMBOHITSILAOZANA 503050001 CEG AMBOHITSILAOZANA 1 671 044 AMBOHITSILAOZANA CEG TANAMBAO JIAPASIKA 622 687 AMPARIHINTSOKATRA 503060013 CEG AMPARIHINTSOKATRA 1 080 499 AMPITATSIMO 503070001 CEG AMPITATSIMO 1 530 936 AMPITATSIMO 503070015 CEG ANNEXE AMBOHITANIBE 860 667 ANDILANATOBY 503080025 CEG ANDRANOKOBAKA 760 039 ANDILANATOBY 503080001 CEG ANDILANATOBY 1 196 620 ANDILANATOBY 503080026 CEG ANNEXE SAHANIDINGANA 709 718 ANDILANATOBY 503080027 CEG COMMUNAUTAIRE AMBODINONOKA 817 973 ANDILANATOBY 503080031 CEG COMMUNAUTAIRE MANGATANY 723 676 ANDILANATOBY 503080036 CEG COMMUNAUTAIRE RANOFOTSY 668 769 ANDROMBA 503090005 CEG ANDROMBA 1 008 043 ANTANANDAVA 503100020 CEG ANTANANDAVA 1 056 579 ANTSANGASANGA 503110004 CEG ANTSANGASANGA 757 763 BEJOFO 503120016 C.E.G. -

Spiny Forest Heterogeneity: Implications for Regeneration and Its Detection
Spiny Forest Heterogeneity: Implications for Regeneration and its Detection Catherine Reuter Advisor: Jules Ramangalahy Academic Director: Jim Hansen Spring 2009 1 Acknowledgements I would like to thank Barry Ferguson for helping me originate the idea for this project and for innumerable resources from compasses to aerial maps. I would also like to thank Christian, translator and invaluable field assistant, without whose innovative thinking and possibly photographic memory this project would not of succeeded. 2 Table of Contents Section Page 88 Acknowledgements ________________________________________________ 2 Abstract __________________________________________________________ 4 Introduction _______________________________________________________ 5 Methods __________________________________________________________ 7 Results ___________________________________________________________ 12 Discussion ________________________________________________________ 17 Conclusion ________________________________________________________ 26 Appendix 1: Comprehensive Species List ________________________________ 27 Appendix 2: FTM 1954 Map of Forest Cover _____________________________ 30 Sources Cited _______________________________________________________ 31 3 ABSTRACT This study sought to verify claims made in a recently published paper by Thomas Elmqvist that certain portions of Madagascar’s spiny forest are rapidly regenerating. The study took place in the forest around the village of Manavy located in Central Antandroy, where historical and current -

Mt Mabu, Mozambique: Biodiversity and Conservation
Darwin Initiative Award 15/036: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems MT MABU, MOZAMBIQUE: BIODIVERSITY AND CONSERVATION November 2012 Jonathan Timberlake, Julian Bayliss, Françoise Dowsett-Lemaire, Colin Congdon, Bill Branch, Steve Collins, Michael Curran, Robert J. Dowsett, Lincoln Fishpool, Jorge Francisco, Tim Harris, Mirjam Kopp & Camila de Sousa ABRI african butterfly research in Forestry Research Institute of Malawi Biodiversity of Mt Mabu, Mozambique, page 2 Front cover: Main camp in lower forest area on Mt Mabu (JB). Frontispiece: View over Mabu forest to north (TT, top); Hermenegildo Matimele plant collecting (TT, middle L); view of Mt Mabu from abandoned tea estate (JT, middle R); butterflies (Lachnoptera ayresii) mating (JB, bottom L); Atheris mabuensis (JB, bottom R). Photo credits: JB – Julian Bayliss CS ‒ Camila de Sousa JT – Jonathan Timberlake TT – Tom Timberlake TH – Tim Harris Suggested citation: Timberlake, J.R., Bayliss, J., Dowsett-Lemaire, F., Congdon, C., Branch, W.R., Collins, S., Curran, M., Dowsett, R.J., Fishpool, L., Francisco, J., Harris, T., Kopp, M. & de Sousa, C. (2012). Mt Mabu, Mozambique: Biodiversity and Conservation. Report produced under the Darwin Initiative Award 15/036. Royal Botanic Gardens, Kew, London. 94 pp. Biodiversity of Mt Mabu, Mozambique, page 3 LIST OF CONTENTS List of Contents .......................................................................................................................... 3 List of Tables ............................................................................................................................. -

Mistletoes on Mmahgh J) Introduced Trees of the World Agriculture
mmAHGH J) Mistletoes on Introduced Trees of the World Agriculture Handbook No. 469 Forest Service U.S. Department of Agriculture Mistletoes on Introduced Trees of the World by Frank G. Hawksworth Plant Pathologist Rocky Mountain Forest and Range Experiment Station Agriculture Handbook No. 469 Forest Service U.S. Department of Agriculture December 1974 CONTENTS Page Introduction ^ Mistletoes and Hosts 3 Host Index of Mistletoes 27 Literature Cited ^^ Library of Congress Catalog No. 74-600182 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington B.C. 20402 Price 75 cents Stock Number 0100-03303 MISTLETOES ON INTRODUCED TREES OF THE WORLD INTRODUCTION Spaulding (1961) published the first attempt at a worldwide inven- tory of the diseases of foreign (introduced) trees of the world. With the widespread introduction of trees to many parts of the world, it is becoming of increasing importance to know the susceptibility of trees introduced to new disease situations. Spaulding's comprehensive lists included forest tree diseases caused by fungi, bacteria, and viruses, but not the mistletoes. Therefore this publication on the mistletoes was prepared to supplement his work. Spaulding considered only forest trees, but the coverage here is expanded to include mistletoes parasitic on introduced forest, crop, orchard, and ornamental trees. In some instances, mistletoes are reported on trees cultivated within different parts of a country where the tree is native. Such records are included if it is indicated in the publication that the mistletoe in question is on planted trees. "Mistletoe" as used in this paper refers to any member of the fam- ilies Loranthaceae or Viscaceae. -

An Invasive Species Spread by Threatened Diurnal Lemurs Impacts Rainforest Structure in Madagascar
Biol Invasions https://doi.org/10.1007/s10530-020-02293-7 (0123456789().,-volV)( 0123456789().,-volV) ORIGINAL PAPER An invasive species spread by threatened diurnal lemurs impacts rainforest structure in Madagascar Camille M. M. DeSisto . Daniel S. Park . Charles C. Davis . Veronarindra Ramananjato . Jadelys L. Tonos . Onja H. Razafindratsima Received: 19 December 2019 / Accepted: 5 June 2020 Ó Springer Nature Switzerland AG 2020 Abstract Invasive species are a major threat to unexplored. By surveying multiple sites across Mada- biodiversity and ecosystem function. Thus, under- gascar’s eastern rainforests, we demonstrate that the standing their spread and ecological impacts is critical introduction of P. cattleianum significantly correlates for management and control. Strawberry guava (Psid- with changes in forest structure—namely tree/shrub ium cattleianum Sabine) is an aggressive invader size, taxonomic richness, and taxonomic diversity. across the tropics and has been rapidly spreading Further, at a local scale, the presence of P. cattleianum throughout the eastern rainforests of Madagascar. was associated with an increase in frugivore species However, both the mechanisms of its spread on the richness; its primary dispersers during our study island and the consequences of its invasion on native period were lemurs. Moreover, we identified species- floral and faunal communities remain largely specific effects of lemur gut-passage on the germina- tion of P. cattleianum seeds. Finally, microsatellite analysis of P. cattleianum from a variety of locations Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10530-020-02293-7) con- across Madagascar demonstrated three distinct, highly tains supplementary material, which is available to authorized differentiated, genetic population clusters, each with users. -
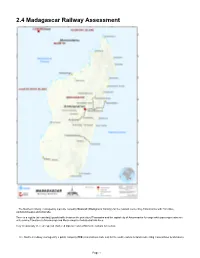
2.4 Madagascar Railway Assessment
2.4 Madagascar Railway Assessment - The Northern railway, managed by a private company Madarail (Madagascar Railway) for the network connecting Antananarivo with Tamatave, Ambatondrazaka and Antsirabe. There is a regular (at least daily) goods traffic between the port city of Toamasina and the capital city of Antananarivo for cargo while passenger trains are only serving Tamatave to Moramanga and Moramanga to Ambatrodrazaka lines. Very occasionally there are special chartered trips on restored Micheline railcars for tourists. - The Southern railway, managed by a public company FCE (Fianarantsoa Cote Est) for the south eastern network connecting Fianarantsoa to Manakara. Page 1 The southern line has regular passenger and cargo trains, which provides a slow but picturesque alternative to the recently rehabilitated road in the region. For more information on railway company contact details, please see the following link: Madagascar Railway Assessment Railway Companies and Consortia 4.2.7 Madagascar Railway Company Contact List Northern railway*: *During our study, Madarail was in the midst of restructuring, therefore, they did not want to share information, statistics or even contacts. All the information gathered and shared in this document comes exclusively from third parties or from data found on the internet. Madarail, was founded on October 10, 2002 following the decision of the Malagasy State to privatize the Malagasy National Railway Network1 (RNCFM). A concession agreement for the management of the North network is then established between the new private operator and the State. Madarail began operating the Northern railway network in Madagascar on 1 July 2003. In 2008, the Belgian operator Vecturis, already active in eight other African countries, became the majority shareholder of the company and the new railway operator. -

Seed Dispersal and Frugivory: Ecological Consequences for Tree
Seed Dispersal and Frugivory: Ecological Consequences for Tree Populations and Bird Communities Von der Fakultät für Mathematik, Informatik und Naturwissenschaften - Fachbereich 1 - der Rheinisch - Westfälischen Technischen Hochschule Aachen zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Diplom-Biologin Bärbel Bleher aus Urach, jetzt Bad Urach Berichter: Universitätsprofessor Dr. rer. nat. Ingolf Schuphan Universitätsprofessor Dr. rer. nat. Hermann Wagner Tag der mündlichen Prüfung: 13. September 2000 If I know a song of Africa, of the giraffe and the African new moon lying on her back, of the plows in the fields and the sweaty faces of the coffee pickers, does Africa know a song of me? Will the air over the plain quiver with a color that I have had on, or the children invent a game in which my name is, or the full moon throw a shadow over the gravel of the drive that was like me, or will the eagles of the Ngong Hills look out for me? T. Blixen dedicated to my parents CONTENTS 1. GENERAL INTRODUCTION 1 1.2 SEED DISPERSAL BY ANIMALS AND CONSEQUENCES FOR PLANTS 1 1.2 FRUIT AVAILABILITY AND CONSEQUENCES FOR FRUGIVOROUS ANIMALS 2 1.3 RELEVANCE FOR CONSERVATION 3 1.4 AIMS OF THESIS 4 2. SEED DISPERSAL BY BIRDS IN A SOUTH AFRICAN AND A MALAGASY COMMIPHORA SPECIES 7 2.1 INTRODUCTION 7 2.2 THE TREES 8 2.3 STUDY SITES 9 2.4 METHODS 10 2.4.1 FRUGIVORE DIVERSITY 10 2.4.2 TREE OBSERVATIONS 10 2.4.3 FRUIT TRAPS 10 2.5 RESULTS 11 2.6 DISCUSSION 16 2.7 SUMMARY 19 3.