•Italian Journal 2006 N. 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
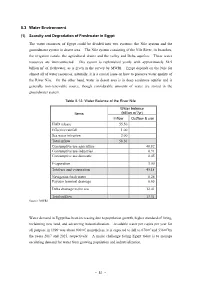
5.3 Water Environment
5.3 Water Environment (1) Scarcity and Degradation of Freshwater in Egypt The water resources of Egypt could be divided into two systems; the Nile system and the groundwater system in desert area. The Nile system consisting of the Nile River, its branches, the irrigation canals, the agricultural drains and the valley and Delta aquifers. These water resources are interconnected. This system is replenished yearly with approximately 58.5 billion m3 of freshwater, as is given in the survey by MWRI. Egypt depends on the Nile for almost all of water resources; naturally, it is a crucial issue on how to preserve water quality of the River Nile. On the other hand, water in desert area is in deep sandstone aquifer and is generally non-renewable source, though considerable amounts of water are stored in the groundwater system. Table 5.13: Water Balance of the River Nile Water balance 3 Items (billion m /yr) Inflow Outflow & use HAD release 55.50 Effective rainfall 1.00 Sea water intrusion 2.00 Total inflow 58.50 Consumptive use agriculture 40.82 Consumptive use industries 0.91 Consumptive use domestic 0.45 Evaporation 3.00 Total use and evaporation 45.18 Navigation fresh water 0.26 Fayoum terminal drainage 0.65 Delta drainage to the sea 12.41 Total outflow 13.31 Source: MWRI Water demand in Egypt has been increasing due to population growth, higher standard of living, reclaiming new land, and advancing industrialization. Available water per capita per year for all purpose in 1999 was about 900m3; nonetheless, it is expected to fall to 670m3 and 536m3 by the years 2017 and 2025, respectively. -

Arab Republic of Egypt Land Reclamation Subsector Review
ReporlNo. 8047.EGT * Arab Republicof Egypt Land ReclamationSubsector Review February1,1990 Public Disclosure Authorized Europe,Middle East and North Africa Region CountryDepartment IlIl AgricultureOperations Division FOR OFFICIALUSE ONLY -~~~~~~~~~~~~~~~~~~~~~~~~~~~~I- .~~~~~~~~~~~~~~~~~~~~~~~ Public Disclosure Authorized .,~~~~~~~~~~~~~~~~~~~~9 .~~~~~~~~~~. 5~ ~ ~ ~~~~~~~~~~ .3 . (, Public Disclosure Authorized ~ ~~~~~~~~~~~~~~~~~ ., ' C' /; .W Ž5 ,(5.t (')'. J C,~~~~~~~~~~~~~a * fE~~~~~~~~~~~~~~~~~'0 ; DocumnentoftheWorldBank PThisdocument has a restricted'distributionand maybe usedjy ,recipientsl -'. ' . ' onlyin theperformance of their official dities. Its contents nay ihototherwise . '. , Public Disclosure Authorized -: r sd w1thtoutWork Btankauthorization. 9' O . , f - ss -, ~ ~ '.9o ~ ~ N . 5f ot < a (- ' '., \;, o ' '~~~~~. >9t I Currency Eguivalents Currency Unit - Egyptian Pound (LE) LE 1.00 - US$ 0.408 US$1.00 - LE 2.45 Weifhts and Measures 1 feddan (fed) - 1.038 acres 1 feddan (fed) - 0.420 hectares (ha) 1 hectare (ha) - 2.380 feddans (fed) m3 - cubic meters Mm3 - million cubic meters Bm3 - billion cubic meters Principal Abbreviations and Acronyms Used ARC Agricultural Research Center BDAC Bank for Development and Agricultu:al Credit DRI Drainage Research Institute EARIS Egyptian American Rural Improvement Service EAUDRL Egyptian Authority for the Utilization and Development of Reclaimed Land ' EEA Electricity and Energy Authority FAO Food and Agriculture Organization GADD General Authority for Desert Development GARPAD General -

Non-Technical Summary Environmental and Social Impact Assessment (ESIA) Report
Arab Republic of Egypt Ministry of Housing, Utilities and Urban Communities European Investment Bank L’Agence Française de Développement (AFD) Construction Authority for Potable Water & Wastewater CAPW Helwan Wastewater Collection & Treatment Project Non-Technical Summary Environmental and Social Impact Assessment (ESIA) Report Date of issue: May 2020 Consulting Engineering Office Prof. Dr.Moustafa Ashmawy Helwan Wastewater Collection & Treatment Project NTS ESIA Report Non - Technical Summary 1- Introduction In Egypt, the gap between water and sanitation coverage has grown, with access to drinking water reaching 96.6% based on CENSUS 2006 for Egypt overall (99.5% in Greater Cairo and 92.9% in rural areas) and access to sanitation reaching 50.5% (94.7% in Greater Cairo and 24.3% in rural areas) according to the Central Agency for Public Mobilization and Statistics (CAPMAS). The main objective of the Project is to contribute to the improvement of the country's wastewater treatment services in one of the major treatment plants in Cairo that has already exceeded its design capacity and to improve the sanitation service level in South of Cairo at Helwan area. The Project for the ‘Expansion and Upgrade of the Arab Abo Sa’ed (Helwan) Wastewater Treatment Plant’ in South Cairo will be implemented in line with the objective of the Egyptian Government to improve the sanitation conditions of Southern Cairo, de-pollute the Al Saff Irrigation Canal and improve the water quality in the canal to suit the agriculture purposes. This project has been identified as a top priority by the Government of Egypt (GoE). The Project will promote efficient and sustainable wastewater treatment in South Cairo and expand the reclaimed agriculture lands by upgrading Helwan Wastewater Treatment Plant (WWTP) from secondary treatment of 550,000 m3/day to advanced treatment as well as expanding the total capacity of the plant to 800,000 m3/day (additional capacity of 250,000 m3/day). -

Inventory of Municipal Wastewater Treatment Plants of Coastal Mediterranean Cities with More Than 2,000 Inhabitants (2010)
UNEP(DEPI)/MED WG.357/Inf.7 29 March 2011 ENGLISH MEDITERRANEAN ACTION PLAN Meeting of MED POL Focal Points Rhodes (Greece), 25-27 May 2011 INVENTORY OF MUNICIPAL WASTEWATER TREATMENT PLANTS OF COASTAL MEDITERRANEAN CITIES WITH MORE THAN 2,000 INHABITANTS (2010) In cooperation with WHO UNEP/MAP Athens, 2011 TABLE OF CONTENTS PREFACE .........................................................................................................................1 PART I .........................................................................................................................3 1. ABOUT THE STUDY ..............................................................................................3 1.1 Historical Background of the Study..................................................................3 1.2 Report on the Municipal Wastewater Treatment Plants in the Mediterranean Coastal Cities: Methodology and Procedures .........................4 2. MUNICIPAL WASTEWATER IN THE MEDITERRANEAN ....................................6 2.1 Characteristics of Municipal Wastewater in the Mediterranean.......................6 2.2 Impact of Wastewater Discharges to the Marine Environment........................6 2.3 Municipal Wasteater Treatment.......................................................................9 3. RESULTS ACHIEVED ............................................................................................12 3.1 Brief Summary of Data Collection – Constraints and Assumptions.................12 3.2 General Considerations on the Contents -

Country Report: Egypt
GLOBAL FOREST RESOURCES ASSESSMENT 2015 COUNTRY REPORT Egypt Rome, 2014 FAO, at the request of its member countries, regularly monitors the world´s forests and their management and uses through the Global Forest Resources Assessment (FRA). This country report is prepared as a contribution to the FAO publication, the Global Forest Resources Assessment 2015 (FRA 2015). The content and the structure are in accordance with the recommendations and guidelines given by FAO in the document Guide for country reporting for FRA 2015 (http://www.fao.org/3/a-au190e.pdf). These reports were submitted to FAO as official government documents. The content and the views expressed in this report are the responsibility of the entity submitting the report to FAO. FAO may not be held responsible for the use which may be made of the information contained in this report. FRA 2015 – Country Report, Egypt TABLE OF CONTENTS Report preparation and contact persons...............................................................................................................................4 1. What is the area of forest and other wooded land and how has it changed over time? ................................................. 5 2. What is the area of natural and planted forest and how has it changed over time? ..................................................... 13 3. What are the stocks and growth rates of the forests and how have they changed? .....................................................17 4. What is the status of forest production and how has it changed over time? .................................................................27 5. How much forest area is managed for protection of soil and water and ecosystem services? ..................................... 34 6. How much forest area is protected and designated for the conservation of biodiversity and how has it changed over time? .................................................................................................................................................................................. -

Egypt State of Environment Report 2008
Egypt State of Environment Report Egypt State of Environment Report 2008 1 Egypt State of Environment Report 2 Egypt State of Environment Report Acknowledgment I would like to extend my thanks and appreciation to all who contributed in producing this report whether from the Ministry,s staff, other ministries, institutions or experts who contributed to the preparation of various parts of this report as well as their distinguished efforts to finalize it. Particular thanks go to Prof. Dr Mustafa Kamal Tolba, president of the International Center for Environment and Development; Whom EEAA Board of Directors is honored with his membership; as well as for his valuable recommendations and supervision in the development of this report . May God be our Guide,,, Minister of State for Environmental Affairs Eng. Maged George Elias 7 Egypt State of Environment Report 8 Egypt State of Environment Report Foreword It gives me great pleasure to foreword State of Environment Report -2008 of the Arab Republic of Egypt, which is issued for the fifth year successively as a significant step of the political environmental commitment of Government of Egypt “GoE”. This comes in the framework of law no.4 /1994 on Environment and its amendment law no.9/2009, which stipulates in its Chapter Two on developing an annual State of Environment Report to be submitted to the president of the Republic and the Cabinet with a copy lodged in the People’s Assembly ; as well as keenness of Egypt’s political leadership to integrate environmental dimension in all fields to achieve sustainable development , which springs from its belief that protecting the environment has become a necessary requirement to protect People’s health and increased production through the optimum utilization of resources . -

Of the Anthophora-Species of Egypt
Prof. Dr. H. PRIESNER A REVIEW OF THE ANTHOPHORA-SPECIES OF EGYPT [Hymenoptera : Apidae] A HEVIEW OF THE ANTHOPHORA-SPECIES OF EGYPT (Hymenoptera : Apidae] by Prof. Dr. H. PRIESNER INTRODUCTION Owing to the difficulties I encountered in trying to identify the Egyptian material of Anthophora in the collections of the Cairo and Ain Shams Universities, I had to penetrate more deeply into this matter, especially when I found that quite a number of species of the local collections were no doubt incorrectly named. With the progress of science, in our particular case with the improvements achieved by finding new distinctive characters and especially in the better relative evaluation of those already known, gained by experience and eye training, there lies upon us the bitter task to criticise and correct our late authorities on this subject who actually did all the spade work that enabled us to start on a considerable higher level of knowledge than that having been at their disposal. Apart from the relatively excellent work of KLUG (1845) and a number of species described by SPINOLA and - much later - GRIBODO, most of the taxonomic work on Anthophora of this country was done by H. FRIESE and A. ALFKEN, their work having been mainly based upon the Apid collections of our late A. ANDRES, while my late friend ALFKEN had also examined specimens he received from the Ministry of Agriculture, Mr. A. ALFIERI and the author. ALFKEN had, obviously with the intention of avoiding the creation of synonyms, identified a number of Egyptian species with such of Asiatic origin. -

Quarterly Performance Monitoring Report
.. .. III .. Healthy MotherlHealthy Child Project .. III Quarterly Performance Monitoring Report .. Option Period Quarter Nine .. October 1- December 31, 2003 .. John Snow, Inc. .. Arabic Software Engineering (ArabSoft) Clark Atlanta University .. The Manolf Group, Inc . .. TransCentury Associates In collaboration with The Ministry of Health and Population Cairo, Egypt and USAID/Egypt .. (Contract No. 263-C-OO-98-00041-00) .. January 19,2004 1 i • JSI Quarterly Perfonnance Monitoring Report - HMlHC October 1 - December 31. 2003 • • TABLE OF CONTENTS • TABLE OF CONTENTS ----------------------- LIST OF ANNEXES ------------------------11 • LIST OF TABLES --------------------I11 • ACRONYMS -~------------------------V .. INTRODUCTION------------------------------------ MAJOR EVENTS DURING THIS QUARTER -------------------3 - C.10.1 TASK ONE: BASIC PACKAGE OF ESSENTIAL SERVICES ESTABLISHED A.'ID .. STANDARDS DEFINED 7 C.10.2 TASK TWO: PRE! IN-SERVICE TRAINING SYSTEM DESIGl'o'ED TO DISSEMINATE .. STANDARDS TO PUBLIC AND PRJVATE PROVIDERS------------------ 13 C.10.3 TASK THREE: PUBLIC AND PRIVATE PROVIDER PARnlERSHIP \\TTH COMMUNlTIES .. TO DEVELOP AND MANAGE DISTRJCTS PLANS 25 C.10.4 TASK FOUR: MONITORING SYSTEM IN PLACE TO TRACK UnLlZAnON ......m IMPACT .. ANDPROVIDEFEEDBACK-------------------------29 C.I 0.5 TASK FIVE: RESEARCH ACTIVITIES------------------ 31 .. C.1O.7 TASK SEVEN: BETTER SOCIAL COMMUNITY SERVICES---------- 33 C.I 0.10 TASK TEN: SMALL GRANTS PROGRAM -------------------- 41 T C.I 0.11 TASK ELEVEN: COMMODITY PROCUREME,,'T PROGRA~t -

Discharge from Municipal Wastewater Treatment Plants Into Rivers Flowing Into the Mediterranean Sea
UNEP(DEPI)/MED WG. 334/Inf.4/Rev.1 15 May 2009 ENGLISH MEDITERRANEAN ACTION PLAN MED POL Meeting of MED POL Focal Points Kalamata (Greece), 2- 4 June 2009 DISCHARGE FROM MUNICIPAL WASTEWATER TREATMENT PLANTS INTO RIVERS FLOWING INTO THE MEDITERRANEAN SEA UNEP/MAP Athens, 2009 TABLE OF CONTENTS PREFACE .................................................................................................................................1 PART I.......................................................................................................................................3 1. ΑΒOUT THE STUDY............................................................................................................... 3 1.1 Historical Background of the Study .....................................................................................3 1.2 Report on the Municipal Wastewater Treatment Plants in the Mediterranean Coastal Cities .........................................................................................................................................4 1.3 Methodology and Procedures of the present Study ............................................................5 2. MUNICIPAL WASTEWATER IN THE MEDITERRANEAN..................................................... 8 2.1 Characteristics of Municipal Wastewater in the Mediterranean ..........................................8 2.2 Impacts of Nutrients ............................................................................................................9 2.3 Impacts of Pathogens..........................................................................................................9 -

Archaologische Fundstellen Ober- Und Mittelagyptens in Ihrer Geologischen Landschaft*)
Archaologische Fundstellen Ober- und Mittelagyptens in ihrer geologischen Landschaft*) Von KARL W. BUTZER Den festliindischen Sockel Agyptens und den groflten Teil des afrikanischen Kontinents bilden metamorphe Gesteine aus dem Archaikum. Dieses kristalline Fundament wurde in der darauf folgenden Zeit (Paliiozoikum) der allmiihlichen Abtragung preisgegeben, bis schlieJllich die bunten kontinentalen Schuttdecken des Nubischen Sandsteins wiihrend der Kreidezeit ab gelagert wurden. Darauf folgten die miichtigen marinen Kalke der Oberkreide und des Alt tertiiirs, welche die Kalkstein-Deckschichten der Libyschen Wiiste bilden. Im Verlauf des Jungtertiiirs (Mioziin und Plioziin var r bis 20 Mill. Jahren) zag sich <las Mittelmeer allmahlich auf seine heutige Kiiste zuriick; sandige und kalkige marine Sedimente dieser Epoche sind auf <las nordlichste Agypten beschriinkt. Wiihrend dies er Zeit ging der Nil, der schon im Alttertiiir angedeutet wird, zur Einschneidung seines jetzigen Tals ins Kalksteinplateau iiber. Nordlich Aswan bestand <las Niltal in seiner jetzigen Lage und in iihnlichem AusmaJl schon an der Grenze Unter-Oberplioziin, als eine erneute Meeresregression in Gestalt eines Meeresgolfs bis nach Oberiigypten talaufwiirts vorgedrungen war. Ablagerungen des oberplioziinen Golfs foll ten den urspriinglichen Taleinschnitt bis etwa I 80 m iiber dem Meeresspiegel. Im Norden sind sie sandig und tonig ausgebildet, wiihrend sich im Siiden Breeden und Konglomerate sowie Kalke und Mergel ablagerten. Zu Beginn des Quartiirs (var I Mill. J ahren) setzte die etappenmiiflige Ausriiumung dieser Golfablagerungen ein, <lurch die dem Tai seine heutige Gestalt verliehen wurde. Die iiber wiegende Tendenz des Flusses ging zur Einschneidung iiber, eine Richtung, die seither nur zeit weise <lurch geringe Aufschotterung und neuerdings Aufschlammung unterbrochen wurde. So bildet die plioziine Fiillung heute nur noch den Taluntergrund, der gelegentlich zutage tritt. -

Phytogeography of the Eastern Desert Flora of Egypt Monier Abd El-Ghani, Fawzy Salama, Boshra Salem, Azza El-Hadidy & Mohamed Abdel-Aleem
Wulfenia 24 (2017): 97–120 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Phytogeography of the Eastern Desert flora of Egypt Monier Abd El-Ghani, Fawzy Salama, Boshra Salem, Azza El-Hadidy & Mohamed Abdel-Aleem Summary: 328 species in total were recorded at 500 sites between 30° 06’ and 24° 00’N in the Eastern Desert of Egypt. The occurrence of species was classified into 5 constancy classes: dominant, very common, common, occasional and sporadic. A sharp decrease in the number of recorded species was noticed along the N–S direction from Cairo-Suez road in the north to Aswan-Baranis road in the south (from 179 to 23), and an increase along the E–W direction from the Red Sea coast in the east to the River Nile Valley in the west (from 46 to 80). It was found out that geographical affinities affect the patterns of species distribution: 82 annual (therophyte) species are dominant life forms within the northern part of the study area, followed by 33 species in the southern part. Phanerophytes (trees) showed a decrease in their number from north (13 species) to south (9 species), but a slight increase from east (9 species) to west (10 species). Distribution maps of local geographical subtypes of each of the 4 major chorotypes are shown and a suggested improved phytogeographical map is presented. Keywords: chorotypes, desert vegetation, distribution maps, Egypt, local subtypes, phytogeography Egypt lies between 22° and 32°N latitude. It is part of the Sahara of North Africa and covers a total area of over one million km2 in the hyperarid region. -
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St.