Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B E N I N Benin
Birnin o Kebbi !( !( Kardi KANTCHARIKantchari !( !( Pékinga Niger Jega !( Diapaga FADA N'GOUMA o !( (! Fada Ngourma Gaya !( o TENKODOGO !( Guéné !( Madécali Tenkodogo !( Burkina Faso Tou l ou a (! Kende !( Founogo !( Alibori Gogue Kpara !( Bahindi !( TUGA Suroko o AIRSTRIP !( !( !( Yaobérégou Banikoara KANDI o o Koabagou !( PORGA !( Firou Boukoubrou !(Séozanbiani Batia !( !( Loaka !( Nansougou !( !( Simpassou !( Kankohoum-Dassari Tian Wassaka !( Kérou Hirou !( !( Nassoukou Diadia (! Tel e !( !( Tankonga Bin Kébérou !( Yauri Atakora !( Kpan Tanguiéta !( !( Daro-Tempobré Dammbouti !( !( !( Koyadi Guilmaro !( Gambaga Outianhou !( !( !( Borogou !( Tounkountouna Cabare Kountouri Datori !( !( Sécougourou Manta !( !( NATITINGOU o !( BEMBEREKE !( !( Kouandé o Sagbiabou Natitingou Kotoponga !(Makrou Gurai !( Bérasson !( !( Boukombé Niaro Naboulgou !( !( !( Nasso !( !( Kounounko Gbangbanrou !( Baré Borgou !( Nikki Wawa Nambiri Biro !( !( !( !( o !( !( Daroukparou KAINJI Copargo Péréré !( Chin NIAMTOUGOU(!o !( DJOUGOUo Djougou Benin !( Guerin-Kouka !( Babiré !( Afekaul Miassi !( !( !( !( Kounakouro Sheshe !( !( !( Partago Alafiarou Lama-Kara Sece Demon !( !( o Yendi (! Dabogou !( PARAKOU YENDI o !( Donga Aledjo-Koura !( Salamanga Yérémarou Bassari !( !( Jebba Tindou Kishi !( !( !( Sokodé Bassila !( Igbéré Ghana (! !( Tchaourou !( !(Olougbé Shaki Togo !( Nigeria !( !( Dadjo Kilibo Ilorin Ouessé Kalande !( !( !( Diagbalo Banté !( ILORIN (!o !( Kaboua Ajasse Akalanpa !( !( !( Ogbomosho Collines !( Offa !( SAVE Savé !( Koutago o !( Okio Ila Doumé !( -

Carte Pédologique De Reconnaissance De La République Populaire Du Bénin À 1/200.000 : Feuille De Djougou
P. FAURE NOTICE EXPLICATIVE No 66 (4) CARTE PEDOLOGIQUE DE RECONNAISSANCE de la République Populaire du Bénin à 1/200.000 Feuille de DJOUGOU OFFICE OE LA RECHERCHE SCIENTIFIWE ET TECHNIOUE OUTRE-MER 1 PARIS 1977 NOTICE EXPLICATIVE No 66 (4) CARTE PEDOLOGIQUE DE RECONNAISSANCE de la RepubliquePopulaire du Bénin à 1 /200.000 Feuille de DJOUGOU P. FAURE ORSTOM PARIS 1977 @ORSTOM 2977 ISBN 2-7099-0423-3(édition cornpl8te) ISBN 2-7099-0433-0 SOMMAI RE l. l INTRODUCTION ........................................ 1 I .GENERALITES SUR LE MILIEU ET LA PEDOGENESE ........... 3 Localisationgéographique ............................ 3 Les conditionsde milieu 1. Le climat ................... 3 2 . La végétation ................. 6 3 . Le modelé et l'hydrographie ....... 8 4 . Le substratum géologique ........ 10 Les matériaux originels et la pédogenèse .................. 12 1 . Les matériaux originels .......... 12 2 . Les processus pédogénétiques ...... 13 II-LESSOLS .......................................... 17 Classification 1. Principes de classification ....... 17 2 . La légende .................. 18 Etudemonographique 1 . Les sols minérauxbruts ......... 20 2 . Les sois peuévolués ............ 21 3 . Les sols ferrugineuxtropicaux ....... 21 4 . Les sols ferraliitiques ........... 38 CONCLUSION .......................................... 43 Répartitiondes' sols . Importance relative . Critèresd'utilisation . 43 Les principalescontraintes pour la mise en valeur ............ 46 BIBLIOGRAPHIE ........................................ 49 1 INTRODUCTION La carte pédologique de reconnaissance à 1/200 000, feuille DJOUGOU, fait partie d'un ensemble de neuf coupures imprimées couvrant la totalité du terri- toire de la République Populaire du Bénin. Les travaux de terrain de la couverture générale ont été effectués de 1967 à 1971 par les quatre pédologues de la Section de Pédologie du Centre O. R.S. T.O.M. de Cotonou : D. DUBROEUCQ, P. FAURE, M. VIENNOT, B. -

The Geography of Welfare in Benin, Burkina Faso, Côte D'ivoire, and Togo
Public Disclosure Authorized Public Disclosure Authorized The Geography of Welfare in Benin, Burkina Faso, Côte d’Ivoire, and Togo Public Disclosure Authorized Nga Thi Viet Nguyen and Felipe F. Dizon Public Disclosure Authorized 00000_CVR_English.indd 1 12/6/17 2:29 PM November 2017 The Geography of Welfare in Benin, Burkina Faso, Côte d’Ivoire, and Togo Nga Thi Viet Nguyen and Felipe F. Dizon 00000_Geography_Welfare-English.indd 1 11/29/17 3:34 PM Photo Credits Cover page (top): © Georges Tadonki Cover page (center): © Curt Carnemark/World Bank Cover page (bottom): © Curt Carnemark/World Bank Page 1: © Adrian Turner/Flickr Page 7: © Arne Hoel/World Bank Page 15: © Adrian Turner/Flickr Page 32: © Dominic Chavez/World Bank Page 48: © Arne Hoel/World Bank Page 56: © Ami Vitale/World Bank 00000_Geography_Welfare-English.indd 2 12/6/17 3:27 PM Acknowledgments This study was prepared by Nga Thi Viet Nguyen The team greatly benefited from the valuable and Felipe F. Dizon. Additional contributions were support and feedback of Félicien Accrombessy, made by Brian Blankespoor, Michael Norton, and Prosper R. Backiny-Yetna, Roy Katayama, Rose Irvin Rojas. Marina Tolchinsky provided valuable Mungai, and Kané Youssouf. The team also thanks research assistance. Administrative support by Erick Herman Abiassi, Kathleen Beegle, Benjamin Siele Shifferaw Ketema is gratefully acknowledged. Billard, Luc Christiaensen, Quy-Toan Do, Kristen Himelein, Johannes Hoogeveen, Aparajita Goyal, Overall guidance for this report was received from Jacques Morisset, Elisée Ouedraogo, and Ashesh Andrew L. Dabalen. Prasann for their discussion and comments. Joanne Gaskell, Ayah Mahgoub, and Aly Sanoh pro- vided detailed and careful peer review comments. -

(DMPA-SC) in Benin
ORIGINAL ARTICLE Introduction of Community-Based Provision of Subcutaneous Depot Medroxyprogesterone Acetate (DMPA-SC) in Benin: Programmatic Results Tishina Okegbe,a Jean Affo,b Florence Djihoun,b Alexis Zannou,b Odilon Hounyo,b Gaston Ahounou,c Karamatou Adegnika Bangbola,c Nancy Harrisd Lay community health workers and facility-based health care providers in Benin were trained to administer DMPA-SC safely and effectively in 10 health zones. Community-based DMPA-SC was popular, particularly among new users of contraception, and could help the country achieve its family planning goals. Résumé en français à la fin de l'article. ABSTRACT The Republic of Benin faces high maternal, newborn, and child mortality; low modern contraceptive use; and a critical shortage of health workers. In 2013, the Government of Benin made 3 reproductive health commitments to improve national health indicators, in- cluding expanding provision of family planning services at the community level through task sharing. Since 2016, the Advancing Partners & Communities (APC) project has been helping the Benin Ministry of Health (MOH) provide subcutaneous depot medroxypro- gesterone acetate (DMPA-SC; brand name Sayana Press) through facility-based health care providers and community health workers known as relais communautaires (RCs). DMPA-SC is an easy-to-administer, discreet injectable contraceptive that provides 3 months of protection from pregnancy. Beginning in May 2017, the government introduced DMPA-SC through a phased approach in 10 health zones, which encompassed 149 health centers and 614 villages. Between June 2017 and June 2018, the MOH and APC trained 278 facility-based providers and 917 RCs to provide DMPA-SC, and nearly 11,000 doses were subsequently administered to 7,997 women at facilities and in communities. -

Processus De Recrutement Des Amod
1 Processus de Recrutement des AMOd 001/2016 Le Gouvernement de la République du Bénin a obtenu, du Fonds Mondial pour l’Assainissement (GSF) une subvention de 5 millions de dollar US, pour les cinq ans de mise en œuvre du Programme d’amélioration de l’accès à l’Assainissement et aux Pratiques d’Hygiène en milieu Rural (PAPHyR). L’objectif global de ce programme est de favoriser l’accès durable et équitable des populations démunies des zones rurales aux services d’assainissement, avec de bonnes pratiques d’hygiène en vue d’accélérer l’amélioration de la santé et de la qualité du cadre de vie des communautés pour l’atteinte des cibles OMD et post OMD. Medical Care Development International (MCDI), SELECTION DES 14 COMMUNES POUR LA MISE l’Agence d’Exécution (AE) du programme, met à EN OEUVRE DU PREMIER TOUR DE disposition une partie de ces fonds pour FINANCEMENT subventionner des ONG ou des Associations en qualité d’Agence de Mise en Œuvre déléguée (AMOd) pour l’exécution des activités du Suite à la publication de l’avis n° programme dans les vingt-sept (27) communes de 01/2015/PAPHyR-MCDI relatif à l’Appel à sa zone d’intervention. Manifestation d’Intérêt (AMI) le 17 août 2015 dans le quotidien ‘’La Nation’’, les 27 communes MCDI dispose à ce titre d’une enveloppe de d’intervention du PAPHyR ont envoyé à l’AE leur 1 100 000 dollars US de subvention (pour le dossier de manifestation d’intérêt pour le suivi des premier tour de financement) à répartir dans les activités du programme dans leurs communes quatre (4) départements d’intervention du respectives. -

Les Communes Du Benin En Chiffres
REPUBLIQUE DU BENIN Fraternité – Justice -Travail ********** COMMISSION NATIONALE DES FINANCES LOCALES ********** SECRETARIAT PERMANENT LES COMMUNES DU BENIN EN CHIFFRES 2010 IMAGE LES COMMUNES DU BENIN EN CHIFFRES 2010 1 Préface Les collectivités territoriales les ressources liées à leurs compétences et jusque- un maillon important dans le ministériels, participe de cette volonté de voir les développementdécentralisées sontde la Nation. aujourd’hui La communeslà mises disposer en œuvre de ressources par les départements financières volonté de leur mise en place suffisantes pour assumer la plénitude des missions qui sont les leurs. des Forces Vives de la Nation de février 1990. Les Si en 2003, au début de la mise en effective de la articles 150, 151,remonte 152 et 153à l’historique de la Constitution Conférence du 11 décembre 1990 a posé le principe de leur libre aux Communes représentaient environ 2% de leursdécentralisation, recettes de les fonctionnementtransferts financiers et de5% l’Etat en décentralisation dont les objectifs majeurs sont la promotionadministration de consacrantla démocratie ainsi l’avènementà la base etde lela transferts ont sensiblement augmenté et développement local. représententmatière d’investissement, respectivement 13%aujourd’hui et 73%. En cesdix Depuis dix (10) ans déjà, nos collectivités administration de leur territoire, assumant ainsi environ quatreans vingtd’expérience (80) milliards en matière de F CFA deen lesterritoriales missions quivivent sont l’apprentissage les leurs. Ces missionsde la libre ne dehorsdécentralisation, des interventions l’Etat a transféré directes auxréalisées communes dans sont guère aisées surtout au regard des ces communes. multiples et pressants besoins à la base, face aux ressources financières souvent limitées. doiventCes efforts en prendrede l’Etat la qui mesure se poursuivront pour une utilisation sans nul YAYI Bon a bien pri sainedoute etméritent transparente d’être de salués ces ressources. -

Evaluation Design Report for the Benin Power Compact's Electricity Generation Project and Electricity Distribution Project
FINAL REPORT Evaluation Design Report for the Benin Power Compact's Electricity Generation Project and Electricity Distribution Project March 7, 2018 Christopher Ksoll Kristine Bos Arif Mamun Anthony Harris Sarah Hughes Submitted to: Millennium Challenge Corporation 1099 14th Street, NW Suite 700 Washington, DC 20005 Project Monitor: Hamissou Samari Contract Number: MCC-16-CON-0058 Submitted by: Mathematica Policy Research 1100 1st Street, NE, 12th Floor Washington, DC 20002-4221 Telephone: (202) 484-9220 Facsimile: (202) 863-1763 Project Director: Sarah Hughes Reference Number: 50339.01.200.031.000 This page has been left blank for double-sided copying. BENIN ENERGY II: EVALUATION DESIGN REPORT MATHEMATICA POLICY RESEARCH CONTENTS ACRONYMS ............................................................................................................................................... VII I. INTRODUCTION ........................................................................................................................................ 1 II. OVERVIEW OF THE COMPACT, GENERATION ACTIVITY, AND DISTRIBUTION ACTIVITY ......................................................................................................................................... 5 A. Overview of the Benin Power Compact ..................................................................................... 5 B. Overview of the theory of change .............................................................................................. 9 C. Economic rate of return and -
Benin Programme (HRDP)
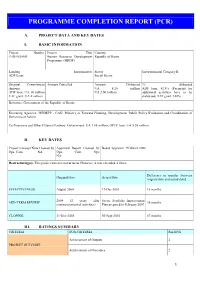
PROGRAMME COMPLETION REPORT (PCR) A. PROJECT DATA AND KEY DATES I. BASIC INFORMATION Project Number Project Title Country P-BJ-IZ0-001 Human Resource Development Republic of Benin Programme (HRDP) Lending Instrument(s) Sector Environmental Category II ADF Loan Social Sector Original Commitment Amount Cancelled Amount Disbursed % disbursed Amount UA 8.28 million ADF loan: 82.8% (Payments for ADF loan: UA 10 million UA 2.00 million additional activities have to be TAF grant: UA 2 million disbursed) TAF grant: 100% Borrower: Government of the Republic of Benin Executing Agencies: MPDEPP - CAG: Ministry of Forward Planning, Development, Public Policy Evaluation and Coordination of Government Action Co-financiers and Other External Partners: Government: UA 1.88 million, OPEC loan: UA 5.58 million II. KEY DATES Project Concept Note Cleared by Appraisal Report Cleared by Board Approval: 15 March 2000 Ops. Com. NA Ops. Com. Ops NA Restructuring(s): This project was not restructured. However, it was extended 4 times. Difference in months [between Original Date Actual Date original date and actual date] EFFECTIVENESS August 2000 17-Dec-2001 16 months 2004 (2 years after Sector Portfolio Improvement MID-TERM REVIEW 36 months commencement of activities) Plan prepared in February 2007 CLOSING 31-Dec-2005 30-Sept-2010 57 months III. RATINGS SUMMARY CRITERIA SUB-CRITERIA RATING Achievement of Outputs 2 PROJECT OUTCOME Achievement of Outcomes 2 1 Timeliness 1 OVERALL PROJECT OUTCOME 2 Design and Readiness 3 BANK PERFORMANCE Supervision 2 OVERALL BANK PERFORMANCE 2 Design and Readiness 2 BORROWER PERFORMANCE Execution 2 OVERALL BORROWER PERFORMANCE 2 IV. RESPONSIBLE BANK STAFF POSITIONS AT APPROVAL AT COMPLETION Regional Director N/A Mr. -

Laws of Attraction Northern Benin and Risk of Violent Extremist Spillover
Laws of Attraction Northern Benin and risk of violent extremist spillover CRU Report Kars de Bruijne Laws of Attraction Northern Benin and risk of violent extremist spillover Kars de Bruijne CRU Report June 2021 This is a joint report produced by the Conflict Research Unit of Clingendael – the Netherlands Institute of International Relations in partnership with the Armed Conflict Location & Event Data Project (ACLED). June 2021 © Netherlands Institute of International Relations ‘Clingendael’. Cover photo: © Julien Gerard Unauthorized use of any materials violates copyright, trademark and / or other laws. Should a user download material from the website or any other source related to the Netherlands Institute of International Relations ‘Clingendael’, or the Clingendael Institute, for personal or non-commercial use, the user must retain all copyright, trademark or other similar notices contained in the original material or on any copies of this material. Material on the website of the Clingendael Institute may be reproduced or publicly displayed, distributed or used for any public and non-commercial purposes, but only by mentioning the Clingendael Institute as its source. Permission is required to use the logo of the Clingendael Institute. This can be obtained by contacting the Communication desk of the Clingendael Institute ([email protected]). The following web link activities are prohibited by the Clingendael Institute and may present trademark and copyright infringement issues: links that involve unauthorized use of our logo, framing, inline links, or metatags, as well as hyperlinks or a form of link disguising the URL. About the author Kars de Bruijne is a Senior Research Fellow with the Clingendael’s Conflict Research Unit and a former Senior Researcher at ACLED. -

Terms of Reference
Telephone: + 49 (0) 228 815 2800 Fax: + 49 (0) 228 815 2898/99 Email: [email protected] TERMS OF REFERENCE National consultant – Support to GCF project document design for a gender-responsive LDN Transformative Project - in Benin Consultancy reference number: CCD/20/GM/33 Background The Global Mechanism is an institution of the UNCCD, mandated to assist countries in the mobilization of financial resources from the public and private sector for activities that prevent, control or reverse desertification, land degradation and drought. As the operational arm of the convention, the Global Mechanism supports countries to translate the Convention into action. In September 2015, the United Nations General Assembly adopted the Sustainable Development Goals, including goal 15, which aims to “protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss”. The main expected result is defined under target 15.3 to “combat desertification, restore degraded land and soil, including land affected by desertification, drought and floods, and strive to achieve a land degradation-neutral world” by 2030. In September 2017, the 13th session of the Conference of Parties (COP) of the UNCCD emphasized the critical role of Land Degradation Neutrality (LDN) Transformative Projects and Programmes for the implementation of the Convention. This has been reaffirmed at the 14th session of the COP in New Delhi in September 2019. The government of Benin has adopted LDN targets at the highest level in 2017 and is now in the process of designing a gender responsive LDN Transformative Project/Programme to translate its voluntary LDN targets into concrete implementation. -

Partner Country Coverage
IMaD’s Lessons Learned Luis Benavente MD, MS CORE’s webinar August 29, 2012 Insert title here Country Number of health Health workers trained in topics related to malaria diagnosis as of June 2012 total N facility visits trained Baseline Outreach How to TOT and On-the- job Database National External Malaria all assesmt. of training & perform supervi- training data entry, Malaria competency micros- Diagnostic support laboratory sors during mainte- Slide sets, assess- copy and capabilities supervision assessments OTSS OTSS nance NAMS ments RDTs Angola 5 6 2 18 12 0 0 12 26 70 Benin 11 409 2 30 1671 9 0 0 40 1,752 Burundi 18 0 0 0 0 0 0 0 0 0 DR Congo 0 0 0 23 0 0 0 0 74 97 Ethiopia 4 0 4 0 0 0 13 17 0 34 Ghana 37 1109 37 46 3704 10 10 6 40 3,853 Guinea Con. 11 0 11 0 0 0 0 0 20 31 Kenya 1192 52 1192 66 312 0 0 37 67 1,674 Liberia 8 200 8 35 975 11 0 7 141 1,177 Madagascar 50 31 50 18 18 0 0 0 23 109 Malawi 14 521 14 75 1132 3 0 0 64 1,288 Mali 5 172 5 24 1188 4 0 0 40 1,261 Nigeria 40 0 0 0 0 0 0 26 0 26 Zambia 6 278 6 12 1733 5 0 9 92 1,857 Total 1,401 2,778 1,331 347 10,745 42 23 114 627 13,229 Proportion of febrile episodes caused by Plasmodium falciparum Source: d’Acremont V, Malaria Journal, 2010 Better testing is needed to assess the impact of malaria control interventions Parasitemia prevalence after IRS, countries with at least biennial monitoring 80% Namibia-C Zimbabwe Moz-Maputo 70% 60% 50% 40% 30% 20% 10% 0% 1948 1950 1952 1954 1956 1958 1960 1962 1964 1966 1968 CORE’s Surviving Malaria Pathway ITNs - Bednet use Caretaker recognizes -

Influence of Climatic Factors on Aggression And
Inuence of climatic factors on aggression and infectivity of Anopheles in the districts the Indoor Residual Spray (IRS) in Northern Benin, West Africa. André SOMINAHOUIN ( [email protected] ) Centre de Recherche Entomologique de Cotonou Germain Gil Padonou CREC Rodrigue Landéhou DGAT Albert Sourou Salako CREC Hermann Sagbohan CREC Idelphonse Ahogni CREC Sylvain Lokonon FASEG Razaki Osse CREC Bénoît Assogba CREC Fiacre Agossa CREC Fortuné Dagnon USAID Christophe Houssou CREC Martin C. Akogbéto CREC Research article Keywords: Infectivity, aggression, Climate, Anopheles gambiae(s.l.), IRS, Benin Page 1/26 Posted Date: September 17th, 2019 DOI: https://doi.org/10.21203/rs.2.14494/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 2/26 Abstract Background: Climate variability inuence the diversity and abundance of malaria vectors and thereby on malaria transmission dynamics. Examine its effect on Anopheles parameters involved in transmission may predict the potential malaria hotspot as a right target for its control intervention strategies. Here, we investigated the inuence of meteorological parameters on the aggressiveness and infectivity of Anopheles in two health districts zones where IRS has been extended in Northern Benin. Methods: Mosquito collections were carried out using human landing catches to evaluate rates of aggression and infectivity in twelve villages. Concomitantly, meteorological data from synoptic stations of Benin and neighbouring countries were collected in 2016-2017. Results: The spatial distribution of infective bites of An. gambiae is characterized by an intense aggression in the rural villages of the study area. Analysis of variances showed signicant HBR difference according to the period but not according to the locality.