OM14 Is a Mitochondrial Receptor for Cytosolic Ribosomes That Supports Co-Translational Import Into Mitochondria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bacterial Cell Membrane
BACTERIAL CELL MEMBRANE Dr. Rakesh Sharda Department of Veterinary Microbiology NDVSU College of Veterinary Sc. & A.H., MHOW CYTOPLASMIC MEMBRANE ➢The cytoplasmic membrane, also called a cell membrane or plasma membrane, is about 7 nanometers (nm; 1/1,000,000,000 m) thick. ➢It lies internal to the cell wall and encloses the cytoplasm of the bacterium. ➢It is the most dynamic structure of a prokaryotic cell. Structure of cell membrane ➢The structure of bacterial plasma membrane is that of unit membrane, i.e., a fluid phospholipid bilayer, composed of phospholipids (40%) and peripheral and integral proteins (60%) molecules. ➢The phospholipids of bacterial cell membranes do not contain sterols as in eukaryotes, but instead consist of saturated or monounsaturated fatty acids (rarely, polyunsaturated fatty acids). ➢Many bacteria contain sterol-like molecules called hopanoids. ➢The hopanoids most likely stabilize the bacterial cytoplasmic membrane. ➢The phospholipids are amphoteric molecules with a polar hydrophilic glycerol "head" attached via an ester bond to two non-polar hydrophobic fatty acid tails. ➢The phospholipid bilayer is arranged such that the polar ends of the molecules form the outermost and innermost surface of the membrane while the non-polar ends form the center of the membrane Fluid mosaic model ➢The plasma membrane contains proteins, sugars, and other lipids in addition to the phospholipids. ➢The model that describes the arrangement of these substances in lipid bilayer is called the fluid mosaic model ➢Dispersed within the bilayer are various structural and enzymatic proteins, which carry out most membrane functions. ➢Some membrane proteins are located and function on one side or another of the membrane (peripheral proteins). -

The Endomembrane System and Proteins
Chapter 4 | Cell Structure 121 Endosymbiosis We have mentioned that both mitochondria and chloroplasts contain DNA and ribosomes. Have you wondered why? Strong evidence points to endosymbiosis as the explanation. Symbiosis is a relationship in which organisms from two separate species depend on each other for their survival. Endosymbiosis (endo- = “within”) is a mutually beneficial relationship in which one organism lives inside the other. Endosymbiotic relationships abound in nature. We have already mentioned that microbes that produce vitamin K live inside the human gut. This relationship is beneficial for us because we are unable to synthesize vitamin K. It is also beneficial for the microbes because they are protected from other organisms and from drying out, and they receive abundant food from the environment of the large intestine. Scientists have long noticed that bacteria, mitochondria, and chloroplasts are similar in size. We also know that bacteria have DNA and ribosomes, just like mitochondria and chloroplasts. Scientists believe that host cells and bacteria formed an endosymbiotic relationship when the host cells ingested both aerobic and autotrophic bacteria (cyanobacteria) but did not destroy them. Through many millions of years of evolution, these ingested bacteria became more specialized in their functions, with the aerobic bacteria becoming mitochondria and the autotrophic bacteria becoming chloroplasts. The Central Vacuole Previously, we mentioned vacuoles as essential components of plant cells. If you look at Figure 4.8b, you will see that plant cells each have a large central vacuole that occupies most of the cell's area. The central vacuole plays a key role in regulating the cell’s concentration of water in changing environmental conditions. -

Cell Wall Ribosomes Nucleus Chloroplast Cytoplasm
Cell Wall Ribosomes Nucleus Nickname: Protector Nickname: Protein Maker Nickname: Brain The cell wall is the outer covering of a Plant cell. It is Ribosomes read the recipe from the The nucleus is the largest organelle in a cell. The a strong and stiff and made of DNA and use this recipe to make nucleus directs all activity in the cell. It also controls cellulose. It supports and protects the plant cell by proteins. The nucleus tells the the growth and reproduction of the cell. holding it upright. It ribosomes which proteins to make. In humans, the nucleus contains 46 chromosomes allows water, oxygen and carbon dioxide to pass in out They are found in both plant and which are the instructions for all the activities in your of plant cell. animal cells. In a cell they can be found cell and body. floating around in the cytoplasm or attached to the endoplasmic reticulum. Chloroplast Cytoplasm Endoplasmic Reticulum Nickname: Oven Nickname: Gel Nickname: Highway Chloroplasts are oval structures that that contain a green Cytoplasm is the gel like fluid inside a The endoplasmic reticulum (ER) is the transportation pigment called chlorophyll. This allows plants to make cell. The organelles are floating around in center for the cell. The ER is like the conveyor belt, you their own food through the process of photosynthesis. this fluid. would see at a supermarket, except instead of moving your groceries it moves proteins from one part of the cell Chloroplasts are necessary for photosynthesis, the food to another. The Endoplasmic Reticulum looks like a making process, to occur. -
Endomembrane System
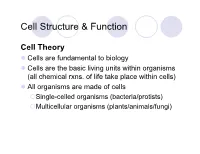
Cell Structure & Function Cell Theory Cells are fundamental to biology Cells are the basic living units within organisms (all chemical rxns. of life take place within cells) All organisms are made of cells Single-celled organisms (bacteria/protists) Multicellular organisms (plants/animals/fungi) Cell Structure & Function Basic Aspects of Cell Structure & Function Plasma membrane Lipid bilayer Proteins DNA-containing region Cytoplasm Eukaryotic v. Prokaryotic cells Prokaryotic v. Eukaryotic Cells Two major classes of cells Prokaryotic cells (pro-, “before”) Cell lacks a “true” nucleus DNA is coiled in a nucleoid region Cells lack nuclear membrane Prokaryotic v. Eukaryotic Cells [attachment structure] [DNA location] [organelles that synthesize proteins] [enclosing the cytoplasm] [rigid structure outside the p.m. ] [jelly-like outer coating] [locomotion organelle] Prokaryotic v. Eukaryotic Cells Eukaryotic cells (eu-, “true”) Nucleus contains most of the cells nuclear material, DNA usually the largest organelle Bordered by a membranous envelope Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Both contain Plasma membrane (functions as a selective barrier) Nucleus (gene-containing organelle) Cytoplasm (region between nucleus and p.m.) Consists of organelles in a fluid (cytosol) Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Organelles Bordered by internal membranes Compartmentalizes the functions of a cell Maintains organelle’s unique environment Most organelles are found in both plant and animal cells Plant v. Animal Cells -

Genic Induction of an Inherited Cytoplasmic Difference
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 29 December 15, 1943 Number ll Copyight 1943 yg the National Academy of Slene GENIC INDUCTION OF AN INHERITED CYTOPLASMIC DIFFERENCE By M. M. RHOADES DEPARTMENT OF BOTANY, COLUMBIA UNIVERSITY Communicated November 16,1943 Although many biologists consider the genes to be the sole determiners of heredity, there are those wbo feel that the cytoplasm contains a system of independent entities which in some cases controls the expression of certain characteristics. The terms genome and plasmone have been used to denote the system of genes and of t-ytoplasmic entities, respec- tively. Although the development of chlorophyll has been shown in hundreds of cases to be under genic control, there are a number of in- stances where chlorophyll variegation is inherited independently of the genome. These chlorophyll variegations, transmitted through the female line only, constitute the most compelling evidence for cytoplasmic inheritance. In these cases the physical entities in the cytoplasm are known to be the plastids; in other cases of plasmatic inheritance the nature of the entities in the cytoplasm can only be conjectured. In maize there are more than one hundred cases where the development of chlorophyll is under genic control. Two examples of cytoplasmically inherited chlorophyll variegation have been reported,1' 2 as has one case of the cytoplasmic inheritance of male sterility.3 Among the chloro- phyll characters in maize which are genically controlled is that of iojap. Maize plants homozygous for the recessive gene iojap (ij) exhibit a chloro- phyll striping or variegation.4 Considerable variation is found in the extent and pattern of the green and white areas of the leaves and culm. -

Cytoplasmic Membrane Systems
Cytoplasmic Membrane Systems Under the light microscope, the cytoplasm of living cells appears relatively devoid of structure. Yet, even before the beginning of the twentieth century, examination of stained sections of animal tissues hinted at the existence of an extensive membrane network within the cytoplasm. It became evident from the early electron microscopic studies and the biochemical investigations that followed that the cytoplasm of eukaryotic cells was subdivided into a variety of distinct compartments bounded by membrane barriers. Just as a house or restaurant is divided into specialized rooms where different activities can take place independent of one another, the cytoplasm of a cell is divided into specialized membranous compartments for analogous reasons. 1 2 The organelles of the endomembrane system are part of a dynamic, integrated network in which materials are shuttled back and forth from one part of the cell to another. For the most part, materials are shuttled between organelles—from the Golgi complex to the plasma membrane, for example—in small, membrane-bounded transport vesicles that bud from a donor membrane compartment. When they reach their destination, the vesicles fuse with the membrane of the acceptor compartment, which receives the vesicle’s soluble cargo as well as its membranous wrapper. Repeated cycles of budding and fusion shuttle a diverse array of materials along numerous pathways that traverse the cell. 3 A biosynthetic pathway can be discerned in which proteins are synthesized in the endoplasmic reticulum, modified during passage through the Golgi complex, and transported from the Golgi complex to various destinations, such as the plasma membrane, a lysosome, or the large vacuole of a plant cell. -

The Centrosome: a Phoenix Organelle of the Immune Response
e Cell Bio gl lo n g i y S Vertii and Doxsey, Single Cell Biol 2016, 5:1 Single-Cell Biology DOI: 10.4172/2168-9431.1000131 ISSN: 2168-9431 Perspective Article Open Access The Centrosome: A Phoenix Organelle of the Immune Response Anastassiia Vertii and Stephen Doxsey* Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, MA 01605, USA Abstract Stress exposure influences the function, quality and duration of an organism’s life. Stresses such as infection can induce inflammation and activate the immune response, which, in turn, protects the organism by eliminating the pathogen. While many aspects of immune system functionality are well established, the molecular, structural and physiological events contributed by the centrosome remain enigmatic. Here we discuss recent advances in the role of the centrosome in the stress response during inflammation and the possible benefits of the centrosome as a stress sensor for the organism. Keywords: Centrosome/Spindle; Pole/Microtubule organizing which surrounds both centrioles and harbors the gamma tubulin ring center (MTOC); Cell stresses; Febrile condition/Fever; Human complexes (γ TURCs) that nucleate the growth of new microtubules [13,14]. The Diversity of Centrosome Locations and Functions Centrosome Responses to and Regulation of Cell The centrosome is a unique organelle in that it is not bounded by membrane like other organelles. The membrane-free status of the Signalling centrosome allows dynamic interactions with the cytoplasm, including Extracellular exposure of the cell to mitogenic factors such as its many molecules and organelles. For example, the centrosome growth hormones activates numerous signaling pathways that, in turn, interacts directly with endosomes to regulate endosome recycling promote cell division. -

1 Introduction to Cell Biology
1 Introduction to cell biology 1.1 Motivation Why is the understanding of cell mechancis important? cells need to move and interact with their environment ◦ cells have components that are highly dependent on mechanics, e.g., structural proteins ◦ cells need to reproduce / divide ◦ to improve the control/function of cells ◦ to improve cell growth/cell production ◦ medical appli- cations ◦ mechanical signals regulate cell metabolism ◦ treatment of certain diseases needs understanding of cell mechanics ◦ cells live in a mechanical environment ◦ it determines the mechanics of organisms that consist of cells ◦ directly applicable to single cell analysis research ◦ to understand how mechanical loading affects cells, e.g. stem cell differentation, cell morphology ◦ to understand how mechanically gated ion channels work ◦ an understanding of the loading in cells could aid in developing struc- tures to grow cells or organization of cells more efficiently ◦ can help us to understand macrostructured behavior better ◦ can help us to build machines/sensors similar to cells ◦ can help us understand the biology of the cell ◦ cell growth is affected by stress and mechanical properties of the substrate the cells are in ◦ understanding mechan- ics is important for knowing how cells move and for figuring out how to change cell motion ◦ when building/engineering tissues, the tissue must have the necessary me- chanical properties ◦ understand how cells is affected by and affects its environment ◦ understand how mechanical factors alter cell behavior (gene expression) -

Cytoplasmic Organization Promotes Protein Diffusion
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.09.451827; this version posted July 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Cytoplasmic organization promotes protein diffusion William Y. C. Huang1, Xianrui Cheng1,2*, and James E. Ferrell, Jr.1,3* 1 Department of Chemical and Systems Biology, Stanford University School of Medicine, Stanford, CA 94305, USA. 2 Present Address: Department of Biological Sciences, University of Southern California, Los Angeles, CA 90089, USA. 3 Department of Biochemistry, Stanford University School of Medicine, Stanford, CA 94305, USA. *Corresponding authors. Email: [email protected] (X.C.); [email protected] (J.E.F.) Author Contributions: W.Y.C.H., X.C., and J.E.F. conceived the study. W.Y.C.H. performed the experiments and analysis. W.Y.C.H., X.C., and J.E.F. interpreted the results. W.Y.C.H., X.C., and J.E.F. wrote the paper. Competing Interest Statement: The authors declare no conflict of interest. 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.09.451827; this version posted July 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT The cytoplasm is highly organized. However, the extent to which this organization influences the dynamics of cytoplasmic proteins is not well understood. Here, we used Xenopus laevis egg extracts as a model system to study diffusion dynamics in organized versus disorganized cytoplasm. -

Centriole Lysosomes Chloroplasts Mitochondrion Endoplasmic Reticulum (ER) Smooth ER Cell Membrane Nucleolus Golgi Body
Virtual Cell Worksheet- ANSWER KEY 1. Centrioles are only found in animal cells. They function in cell division . They have 9 groups of 3 Centriole arrangement of the protein fibers. Draw a picture of a centriole in the box. 2. Lysosomes are called suicide sacks. They are produced by the golgi body. They consist of a single Lysosomes membrane surrounding powerful digestive enzymes. Those lumpy brown structures are digestive enzymes . They help protect you by destroying the bacteria that your white blood cells engulf. Lysosomes act as a clean up crew for the cell. Zoom in and draw what you see. 3. Chloroplasts are the site of photosynthesis . They consist of a double membrane. The stacks of disk Chloroplasts like structures are called the grana . The membranes connecting them are the thylakoid membranes. Zoom in and draw a picture. 4. Mitochondrion is the powerhouse of the cell. It is the site of respiration . It has a double membrane. Mitochondrion The inner membrane is where most aerobic respiration occurs. The inner membrane is ruffled with a very large surface area. These ruffles are called cristae . Mitochondria have their own DNA and manufacture some of their own proteins . Draw a picture of the mitochondrion with its membrane cut. 5. Endoplasmic Reticulum (ER) is a series of double membranes that loop back and forth between the Endoplasmic cell membrane and the nucleus . These membranes fill the cytoplasm but you cannot see them because Reticulum (ER) they are very transparent . The rough E.R. has ribosomes attached to it. This gives it its texture. -

Cell and Cell Division
Cell and Cell Division Chapter 2 Lecture Outline Cell Cell membrane Nucleus: Nuclear Envelope, Nucleoplasm and Chromatin (DNA + Histones) Cytoplasm: Cytosol and Cell Organelles Cell Division Cell Cycle Mitosis: division of nucleus Cytokinesis: division of cytoplasm Cell Theory 4 basic concepts of cell theory are: Cells are the units of structure (building blocks) of all organisms Cells are the smallest unit of function in all organisms Cells originate only from pre-existing cells by cell division. All cells maintain homeostasis (internal conditions within limits) Cell Membrane All cells are covered with a thin covering of a double layer of Phospholipids and associated Proteins present here and there. Each phospholipid has a polar (hydrophilic) head and non-polar (hydrophobic) tails. In the double layer the tails face each other forming a hydrophobic barrier which keeps water dissolved contents inside. Proteins may be Intrinsic – embedded in the lipid double layer and Extrinsic associated outside the lipid double layer. Cytoplasm Cytoplasm is the living fluid part between cell membrane and nucleus. It has special structures called Cell Organelles in it. Cytosol is the liquid part of cytoplasm formed of water having dissolved or suspended substances in it. Cell Organelles are organ like each performing specific function/s but formed of molecules and membranes only (sub-cellular). Double Membrane bound Organelles: Mitochondria, Chloroplasts, Endoplasmic Reticulum, Golgi Body, and Nucleus. Single Membrane bound Organelles: Lysosomes, Peroxisomes, Vacuoles Organelles lacking any membrane: Ribosomes, Centrioles, Nucleolus Nucleus and Ribosomes 1 Genetic Control of the Cell Nucleus: is the most distinct structure inside cell visible with light microscope. -

Cellular Structure and Function Section ●3 Structures and Organelles
chapter 7 Cellular Structure and Function section ●3 Structures and Organelles Before You Read -!). )DEA The eukaryotic cell contains For cells to function correctly, each part must do its job. organelles. Members of families have jobs or chores that help the whole What You’ll Learn family. On the lines below, list your family members and their differences in the structures of jobs. plant and animal cells Read to Learn Identify the Parts Highlight Cytoplasm and Cytoskeleton each cell structure as you read The environment inside the plasma membrane is a about it. Underline the function semifl uid material called cytoplasm. Scientists once thought of each part. the organelles of eukaryotic cells fl oated freely in the cell’s cytoplasm. As technology improved, scientists discovered more about cell structures. They discovered a structure within the cytoplasm called the cytoskeleton. The cytoskeleton is a network of long, thin protein fi bers that provide an anchor for organelles inside the cell. The cell’s shape and movement depend on the cytoskeleton. Two types of protein fi bers make up the cytoskeleton. Microtubules are long, hollow protein cylinders that form a fi rm skeleton for the cell. They assist in moving substances within the cell. Microfi laments are thin protein threads that help give the cell shape and enable the entire cell or parts of the cell to move. 1. Name one cell function that takes place in organelles. Cell Structures Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Companies, a division of The McGraw-Hill © Glencoe/McGraw-Hill, Copyright All chemical processes of a typical eukaryotic cell take place in the organelles, which move around in the cell’s cytoplasm.