Cytoplasmic Membrane Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bacterial Cell Membrane
BACTERIAL CELL MEMBRANE Dr. Rakesh Sharda Department of Veterinary Microbiology NDVSU College of Veterinary Sc. & A.H., MHOW CYTOPLASMIC MEMBRANE ➢The cytoplasmic membrane, also called a cell membrane or plasma membrane, is about 7 nanometers (nm; 1/1,000,000,000 m) thick. ➢It lies internal to the cell wall and encloses the cytoplasm of the bacterium. ➢It is the most dynamic structure of a prokaryotic cell. Structure of cell membrane ➢The structure of bacterial plasma membrane is that of unit membrane, i.e., a fluid phospholipid bilayer, composed of phospholipids (40%) and peripheral and integral proteins (60%) molecules. ➢The phospholipids of bacterial cell membranes do not contain sterols as in eukaryotes, but instead consist of saturated or monounsaturated fatty acids (rarely, polyunsaturated fatty acids). ➢Many bacteria contain sterol-like molecules called hopanoids. ➢The hopanoids most likely stabilize the bacterial cytoplasmic membrane. ➢The phospholipids are amphoteric molecules with a polar hydrophilic glycerol "head" attached via an ester bond to two non-polar hydrophobic fatty acid tails. ➢The phospholipid bilayer is arranged such that the polar ends of the molecules form the outermost and innermost surface of the membrane while the non-polar ends form the center of the membrane Fluid mosaic model ➢The plasma membrane contains proteins, sugars, and other lipids in addition to the phospholipids. ➢The model that describes the arrangement of these substances in lipid bilayer is called the fluid mosaic model ➢Dispersed within the bilayer are various structural and enzymatic proteins, which carry out most membrane functions. ➢Some membrane proteins are located and function on one side or another of the membrane (peripheral proteins). -

A Tour of the Cell Overview
Unit 3: The Cell Name______________________ Chapter 6: A Tour of the Cell Overview 6.1 Biologists use microscopes and the tools of biochemistry to study cells The discovery and early study of cells progressed with the invention of microscopes in 1590 and their improvement in the 17th century. In a light microscope (LM), visible light passes through the specimen and then through glass lenses. ○ The lenses refract light so that the image is magnified into the eye or a camera. Microscopes vary in magnification, resolution, and contrast. ○ Magnification is the ratio of an object’s image to its real size. A light microscope can magnify effectively to about 1,000 times the real size of a specimen. ○ Resolution is a measure of image clarity. It is the minimum distance two points can be separated and still be distinguished as two separate points. The minimum resolution of an LM is about 200 nanometers (nm), the size of a small bacterium. ○ Contrast accentuates differences in parts of the sample. It can be improved by staining or labeling of cell components so they stand out. Although an LM can resolve individual cells, it cannot resolve much of the internal anatomy, especially the organelles, membrane-enclosed structures within eukaryotic cells. The size range of cells 1 To resolve smaller structures, scientists use an electron microscope (EM), which focuses a beam of electrons through the specimen or onto its surface. ○ Theoretically, the resolution of a modern EM could reach 0.002 nm, but the practical limit is closer to about 2 nm. Scanning electron microscopes (SEMs) are useful for studying the surface structure or topography of a specimen. -

The Endomembrane System and Proteins
Chapter 4 | Cell Structure 121 Endosymbiosis We have mentioned that both mitochondria and chloroplasts contain DNA and ribosomes. Have you wondered why? Strong evidence points to endosymbiosis as the explanation. Symbiosis is a relationship in which organisms from two separate species depend on each other for their survival. Endosymbiosis (endo- = “within”) is a mutually beneficial relationship in which one organism lives inside the other. Endosymbiotic relationships abound in nature. We have already mentioned that microbes that produce vitamin K live inside the human gut. This relationship is beneficial for us because we are unable to synthesize vitamin K. It is also beneficial for the microbes because they are protected from other organisms and from drying out, and they receive abundant food from the environment of the large intestine. Scientists have long noticed that bacteria, mitochondria, and chloroplasts are similar in size. We also know that bacteria have DNA and ribosomes, just like mitochondria and chloroplasts. Scientists believe that host cells and bacteria formed an endosymbiotic relationship when the host cells ingested both aerobic and autotrophic bacteria (cyanobacteria) but did not destroy them. Through many millions of years of evolution, these ingested bacteria became more specialized in their functions, with the aerobic bacteria becoming mitochondria and the autotrophic bacteria becoming chloroplasts. The Central Vacuole Previously, we mentioned vacuoles as essential components of plant cells. If you look at Figure 4.8b, you will see that plant cells each have a large central vacuole that occupies most of the cell's area. The central vacuole plays a key role in regulating the cell’s concentration of water in changing environmental conditions. -

Cell Wall Ribosomes Nucleus Chloroplast Cytoplasm
Cell Wall Ribosomes Nucleus Nickname: Protector Nickname: Protein Maker Nickname: Brain The cell wall is the outer covering of a Plant cell. It is Ribosomes read the recipe from the The nucleus is the largest organelle in a cell. The a strong and stiff and made of DNA and use this recipe to make nucleus directs all activity in the cell. It also controls cellulose. It supports and protects the plant cell by proteins. The nucleus tells the the growth and reproduction of the cell. holding it upright. It ribosomes which proteins to make. In humans, the nucleus contains 46 chromosomes allows water, oxygen and carbon dioxide to pass in out They are found in both plant and which are the instructions for all the activities in your of plant cell. animal cells. In a cell they can be found cell and body. floating around in the cytoplasm or attached to the endoplasmic reticulum. Chloroplast Cytoplasm Endoplasmic Reticulum Nickname: Oven Nickname: Gel Nickname: Highway Chloroplasts are oval structures that that contain a green Cytoplasm is the gel like fluid inside a The endoplasmic reticulum (ER) is the transportation pigment called chlorophyll. This allows plants to make cell. The organelles are floating around in center for the cell. The ER is like the conveyor belt, you their own food through the process of photosynthesis. this fluid. would see at a supermarket, except instead of moving your groceries it moves proteins from one part of the cell Chloroplasts are necessary for photosynthesis, the food to another. The Endoplasmic Reticulum looks like a making process, to occur. -

Vesicle Traffic in the Endomembrane System: a Tale of Cops, Rabs and Snares Andreas Nebenführ
507 Vesicle traffic in the endomembrane system: a tale of COPs, Rabs and SNAREs Andreas Nebenführ Recent years have seen remarkable progress in our contrast, mammalian cells have an intermediate recycling understanding of the endomembrane system of plants. A large compartment between the ER and the Golgi, the vesiculo- number of genes and proteins that are involved in membrane tubular cluster (VTC), that does not exist in plants. exchange between the different compartments of this system have been identified on the basis of their similarity to animal The transport of proteins and membranes between the and yeast homologs. These proteins indicate that the different compartments of the endomembrane system is endomembrane system in plants functions in essentially the mediated by small carriers, the transport vesicles. The same way as those in other eukaryotes. However, a growing basic mechanisms involved in membrane-exchange reactions number of examples demonstrate that the dynamic interplay appear to be the same, irrespective of the organelles between membrane-exchange proteins can be regulated involved [2]. The initial step involves the formation of a differently in plant cells. Novel tools and a better understanding proteinaceous membrane coat that plays a two-fold role. of the molecular effects of the inhibitor brefeldin A are helping First, coat proteins deform the membrane so as to form a to unravel these plant-specific adaptations. bud and eventually a vesicle, and second, they are involved in cargo selection. The assembly of the coat is Addresses controlled by a small GTPase of the Ras superfamily, University of Tennessee, Department of Botany and School of which in turn is activated by a specific guanidine-nucleotide Genome Science and Technology, 437 Hesler Biology, Knoxville, exchange factor (GEF). -
Endomembrane System
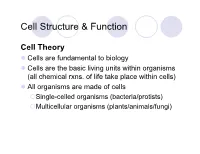
Cell Structure & Function Cell Theory Cells are fundamental to biology Cells are the basic living units within organisms (all chemical rxns. of life take place within cells) All organisms are made of cells Single-celled organisms (bacteria/protists) Multicellular organisms (plants/animals/fungi) Cell Structure & Function Basic Aspects of Cell Structure & Function Plasma membrane Lipid bilayer Proteins DNA-containing region Cytoplasm Eukaryotic v. Prokaryotic cells Prokaryotic v. Eukaryotic Cells Two major classes of cells Prokaryotic cells (pro-, “before”) Cell lacks a “true” nucleus DNA is coiled in a nucleoid region Cells lack nuclear membrane Prokaryotic v. Eukaryotic Cells [attachment structure] [DNA location] [organelles that synthesize proteins] [enclosing the cytoplasm] [rigid structure outside the p.m. ] [jelly-like outer coating] [locomotion organelle] Prokaryotic v. Eukaryotic Cells Eukaryotic cells (eu-, “true”) Nucleus contains most of the cells nuclear material, DNA usually the largest organelle Bordered by a membranous envelope Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Both contain Plasma membrane (functions as a selective barrier) Nucleus (gene-containing organelle) Cytoplasm (region between nucleus and p.m.) Consists of organelles in a fluid (cytosol) Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Organelles Bordered by internal membranes Compartmentalizes the functions of a cell Maintains organelle’s unique environment Most organelles are found in both plant and animal cells Plant v. Animal Cells -

Cell and Cell Division
Cell and Cell Division Chapter 2 Lecture Outline Cell Cell membrane Nucleus: Nuclear Envelope, Nucleoplasm and Chromatin (DNA + Histones) Cytoplasm: Cytosol and Cell Organelles Cell Division Cell Cycle Mitosis: division of nucleus Cytokinesis: division of cytoplasm Cell Theory 4 basic concepts of cell theory are: Cells are the units of structure (building blocks) of all organisms Cells are the smallest unit of function in all organisms Cells originate only from pre-existing cells by cell division. All cells maintain homeostasis (internal conditions within limits) Cell Membrane All cells are covered with a thin covering of a double layer of Phospholipids and associated Proteins present here and there. Each phospholipid has a polar (hydrophilic) head and non-polar (hydrophobic) tails. In the double layer the tails face each other forming a hydrophobic barrier which keeps water dissolved contents inside. Proteins may be Intrinsic – embedded in the lipid double layer and Extrinsic associated outside the lipid double layer. Cytoplasm Cytoplasm is the living fluid part between cell membrane and nucleus. It has special structures called Cell Organelles in it. Cytosol is the liquid part of cytoplasm formed of water having dissolved or suspended substances in it. Cell Organelles are organ like each performing specific function/s but formed of molecules and membranes only (sub-cellular). Double Membrane bound Organelles: Mitochondria, Chloroplasts, Endoplasmic Reticulum, Golgi Body, and Nucleus. Single Membrane bound Organelles: Lysosomes, Peroxisomes, Vacuoles Organelles lacking any membrane: Ribosomes, Centrioles, Nucleolus Nucleus and Ribosomes 1 Genetic Control of the Cell Nucleus: is the most distinct structure inside cell visible with light microscope. -

Endomembrane System– Endoplasmic Reticulum, Golgi Apparatus, Lysosomes, Peroxisomes, Vacuoles, Vesicles
Chapter 7. The Cell: Endomembrane System– Endoplasmic Reticulum, Golgi Apparatus, Lysosomes, Peroxisomes, Vacuoles, Vesicles AP Biology 2005-2006 Overview . Play key role in synthesis (& hydrolysis) of macromolecules in cell . Various “players” modify macromolecules for various functions AP Biology 2005-2006 Endoplasmic Reticulum . Function manufactures membranes & performs many bio-synthesis functions . Structure membrane connected to nuclear envelope & extends throughout cell accounts for 50% membranes in eukaryotic cell . rough ER = bound ribosomes . smooth ER = no ribosomes AP Biology 2005-2006 Types of ER AP Biology 2005-2006 Smooth ER function . Factory processing operations many metabolic processes . synthesis & hydrolysis enzymes of smooth ER… . synthesize lipids, oils, phospholipids, steroids & sex hormones . hydrolysis (breakdown) of glycogen (in liver) into glucose . detoxify drugs & poisons (in liver) ex. alcohol & barbiturates AP Biology 2005-2006 Rough ER function . Produce proteins for export out of cell protein secreting cells packaged into transport vesicles for export which cells have a lot of rough ER? AP Biology 2005-2006 Membrane Factory . Synthesize membrane phospholipids build new membrane as ER membrane expands, bud off & transfer to other parts of cell that need membranes . Synthesize membrane proteins membrane bound proteins synthesized directly into membrane processing to make glycoproteins AP Biology 2005-2006 AP Biology 2005-2006 AP Biology 2005-2006 Golgi Apparatus . Function finishes, sorts, & ships cell products . “shipping & receiving department” center of manufacturing, warehousing, sorting & shipping extensive in cells specialized for secretion which cells have a lot AoP Bf iGoloogylgi? 2005-2006 Golgi Apparatus . Structure flattened membranous sacs = cisternae . look like stack of pita bread 2 sides = 2 functions . cis = receives material by fusing with vesicles = “receiving” . -

Genic Induction of an Inherited Cytoplasmic Difference
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 29 December 15, 1943 Number ll Copyight 1943 yg the National Academy of Slene GENIC INDUCTION OF AN INHERITED CYTOPLASMIC DIFFERENCE By M. M. RHOADES DEPARTMENT OF BOTANY, COLUMBIA UNIVERSITY Communicated November 16,1943 Although many biologists consider the genes to be the sole determiners of heredity, there are those wbo feel that the cytoplasm contains a system of independent entities which in some cases controls the expression of certain characteristics. The terms genome and plasmone have been used to denote the system of genes and of t-ytoplasmic entities, respec- tively. Although the development of chlorophyll has been shown in hundreds of cases to be under genic control, there are a number of in- stances where chlorophyll variegation is inherited independently of the genome. These chlorophyll variegations, transmitted through the female line only, constitute the most compelling evidence for cytoplasmic inheritance. In these cases the physical entities in the cytoplasm are known to be the plastids; in other cases of plasmatic inheritance the nature of the entities in the cytoplasm can only be conjectured. In maize there are more than one hundred cases where the development of chlorophyll is under genic control. Two examples of cytoplasmically inherited chlorophyll variegation have been reported,1' 2 as has one case of the cytoplasmic inheritance of male sterility.3 Among the chloro- phyll characters in maize which are genically controlled is that of iojap. Maize plants homozygous for the recessive gene iojap (ij) exhibit a chloro- phyll striping or variegation.4 Considerable variation is found in the extent and pattern of the green and white areas of the leaves and culm. -

Chapter 6 a Tour of the Cell
Chapter 6 A Tour of the Cell PowerPoint® Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Overview: The Fundamental Units of Life • All organisms are made of cells • The cell is the simplest collection of matter that can live • Cell structure is correlated to cellular function • All cells are related by their descent from earlier cells Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 6-1 Concept 6.1: To study cells, biologists use microscopes and the tools of biochemistry • Though usually too small to be seen by the unaided eye, cells can be complex Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Microscopy • Scientists use microscopes to visualize cells too small to see with the naked eye • In a light microscope (LM), visible light passes through a specimen and then through glass lenses, which magnify the image Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings • The quality of an image depends on – Magnification, the ratio of an object’s image size to its real size – Resolution, the measure of the clarity of the image, or the minimum distance of two distinguishable points – Contrast, visible differences in parts of the sample Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 6-2 10 m Human height 1 m Length -

The Neuronal Endomembrane System III
0270.6474/85/0512-3135$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 12. pp. 3135-3144 Pnnted in U.S.A. December 1985 The Neuronal Endomembrane System III. The Origins of Axoplasmic Reticulum Discrete Axonal Cisternae the Axon Hillock’ JAMES D. LINDSEY2 AND MARK H. ELLISMAN Department of Neurosciences, University of California, San Diego, School of Medicine, La Jolla, California 92093 Abstract The axoplasmic reticulum (AR) and the discrete element vesicles were usually found in close association with the (e.g., vesicles, vesiculotubular bodies, multivesicular bodies, trans face of the Golgi apparatus. These results indirectly etc.) constitute the endomembrane system of the axon. It is support the hypothesis that vectors of fast axonal transport, reported here that the AR of bullfrog sciatic nerve readily fills namely the discrete elements, form directly at the trans face with osmium deposits during osmium impregnation. In con- of the Golgi apparatus. From here they move toward and trast, the discrete elements and mitochondria are highly subsequently down the axon without any membrane fission- resistant to impregnation. Hence this preparation is well fusion events with either RER or AR. AR, although it forms suited to address the nature of possible interactions between continuities with RER, retains a distinctly different chemical AR and rough endoplasmic reticulum (RER) in the axon composition from RER as evidenced by its much higher hillock. It is also ideal to study the origin of the axonal discrete affinity for osmium. Thus, it should be considered as an elements within the cell body as well as their interaction with endomembrane component separate from, although inti- other somal endomembrane system components. -

The Centrosome: a Phoenix Organelle of the Immune Response
e Cell Bio gl lo n g i y S Vertii and Doxsey, Single Cell Biol 2016, 5:1 Single-Cell Biology DOI: 10.4172/2168-9431.1000131 ISSN: 2168-9431 Perspective Article Open Access The Centrosome: A Phoenix Organelle of the Immune Response Anastassiia Vertii and Stephen Doxsey* Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, MA 01605, USA Abstract Stress exposure influences the function, quality and duration of an organism’s life. Stresses such as infection can induce inflammation and activate the immune response, which, in turn, protects the organism by eliminating the pathogen. While many aspects of immune system functionality are well established, the molecular, structural and physiological events contributed by the centrosome remain enigmatic. Here we discuss recent advances in the role of the centrosome in the stress response during inflammation and the possible benefits of the centrosome as a stress sensor for the organism. Keywords: Centrosome/Spindle; Pole/Microtubule organizing which surrounds both centrioles and harbors the gamma tubulin ring center (MTOC); Cell stresses; Febrile condition/Fever; Human complexes (γ TURCs) that nucleate the growth of new microtubules [13,14]. The Diversity of Centrosome Locations and Functions Centrosome Responses to and Regulation of Cell The centrosome is a unique organelle in that it is not bounded by membrane like other organelles. The membrane-free status of the Signalling centrosome allows dynamic interactions with the cytoplasm, including Extracellular exposure of the cell to mitogenic factors such as its many molecules and organelles. For example, the centrosome growth hormones activates numerous signaling pathways that, in turn, interacts directly with endosomes to regulate endosome recycling promote cell division.