Does the Morphology of the Forelimb Flexor Muscles Differ Between Lizards Using Different Habitats?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
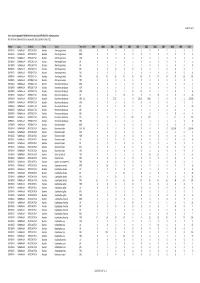
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Evolutionary History of Spiny- Tailed Lizards (Agamidae: Uromastyx) From
Received: 6 July 2017 | Accepted: 4 November 2017 DOI: 10.1111/zsc.12266 ORIGINAL ARTICLE Evolutionary history of spiny- tailed lizards (Agamidae: Uromastyx) from the Saharo- Arabian region Karin Tamar1 | Margarita Metallinou1† | Thomas Wilms2 | Andreas Schmitz3 | Pierre-André Crochet4 | Philippe Geniez5 | Salvador Carranza1 1Institute of Evolutionary Biology (CSIC- Universitat Pompeu Fabra), Barcelona, The subfamily Uromastycinae within the Agamidae is comprised of 18 species: three Spain within the genus Saara and 15 within Uromastyx. Uromastyx is distributed in the 2Allwetterzoo Münster, Münster, Germany desert areas of North Africa and across the Arabian Peninsula towards Iran. The 3Department of Herpetology & systematics of this genus has been previously revised, although incomplete taxo- Ichthyology, Natural History Museum of nomic sampling or weakly supported topologies resulted in inconclusive relation- Geneva (MHNG), Geneva, Switzerland ships. Biogeographic assessments of Uromastycinae mostly agree on the direction of 4CNRS-UMR 5175, Centre d’Écologie Fonctionnelle et Évolutive (CEFE), dispersal from Asia to Africa, although the timeframe of the cladogenesis events has Montpellier, France never been fully explored. In this study, we analysed 129 Uromastyx specimens from 5 EPHE, CNRS, UM, SupAgro, IRD, across the entire distribution range of the genus. We included all but one of the rec- INRA, UMR 5175 Centre d’Écologie Fonctionnelle et Évolutive (CEFE), PSL ognized taxa of the genus and sequenced them for three mitochondrial and three Research University, Montpellier, France nuclear markers. This enabled us to obtain a comprehensive multilocus time- calibrated phylogeny of the genus, using the concatenated data and species trees. We Correspondence Karin Tamar, Institute of Evolutionary also applied coalescent- based species delimitation methods, phylogenetic network Biology (CSIC-Universitat Pompeu Fabra), analyses and model- testing approaches to biogeographic inferences. -

(Reptilia, Agamidae) in Western Sahara: De
Rev. Esp. Herp. ( 1998) 12:97-109 97 Chorological analysis and morphological variations of Saurians of the genus Uromastyx (Reptilia, Agamidae) in western Sabara. Description of two new taxa. 1 2 3 JOSÉ ANTONIO MATE0 ·3, PHILIPPE ÜENIEZ , LUIS FELIPE LÓPEZ-JURAD0 & JACQUES BONS2 I Estación Biológica de Doñana-CSIC, Apartado 1056, E-4108 Sevilla, Spain. 2laboratoire de Biogéographie et Ecologie des Vertébrés-EPHE, Université Montpellier 2, Place Eugene Bataillon, F-34095 Montpellier, France. 3 Departamento de Biología, Universidad de las Palmas de Gran Canaria, 35017 las Palmas de Gran Canaria, Spain. E-mail: luisfelipe. lopez@biologia. ulpgc. es Abstract: The description of a new species ofthe genus Uromastyx is proposed on the basis oftwo specimens from the Adrar Souttouf in Western Sahara. This taxon differs greatly from U. acanthinura on account its larger size, the much larger number of scales, the arrangement of tubercules on its upper thighs, the different habitus and colouring. These morphological features mean it closely resembles U. aegyptia. The existence of a relictual U. aegyptia-group throughout the Sahara is suggested. In addition, the morphological variations in Spiny-tailed agamas (or Mastigures) ofthe Uromastyx acanthinura group in the west ofthe Sahara are briefl y analysed. This produces evidence for the existence of a species proper to Western Sahara and surrounding areas, Uromastyx jlavifasciata, represented by two subspecies: U. f jlavifasciata in the north and U. f obscura subsp. nov. in the south. The latter new form is characterised by uniformly black colouring, even in active individuals. This work also demonstrates that Uromastyx acanthinura werneri does not penetrate Western Sahara and that its distribution is parapatric with that of U. -

Genetic Relationship of Three Butterfly Lizard Species (Leiolepis Reevesii Rubritaeniata, Leiolepis Belliana Belliana, Leiolepis
Kasetsart J. (Nat. Sci.) 44 : 424 - 435 (2010) Genetic Relationship of Three Butterfly Lizard Species (Leiolepis reevesii rubritaeniata, Leiolepis belliana belliana, Leiolepis boehmei, Agamidae, Squamata) Inferred from Nuclear Gene Sequence Analyses Kornsorn Srikulnath1, 2, Kazumi Matsubara3, Yoshinobu Uno2, Amara Thongpan1, Saowanee Suputtitada1, Chizuko Nishida2, 3, Yoichi Matsuda2, 3, 4 and Somsak Apisitwanich1* ABSTRACT The genetic relationship was investigated of three butterfly lizard species (Leiolepis reevesii rubritaeniata, L. belliana belliana and L. boehmei) selectively inhabiting Thailand. The findings were based on RAG1 and C-mos gene analyses. The DNA sequences were also compared with the other squamate reptiles. The analysis strongly supported that L. reevesii rubritaeniata was related more closely to L. belliana belliana than to L. boehmei. The phylogenetic position of Leiolepis spp., however, was contentious with regard to its relationship among the Leiolepidinae, Agaminae and Chamaeleonidae, which suggested that their phylogeny remains uncertain. Keywords: butterfly lizard, Leiolepidinae, phylogeny, RAG1, C-mos INTRODUCTION inhabit Southeast Asia. They show a great variety of karyotypes and sexual systems. In Thailand, The Squamata is the most diverse there are three species, which barely can be reptilian order that has been classified traditionally discriminated from other congeneric species by into three suborders: Serpentes (snakes), their typical scale and skin coloration (Peters, Amphisbaenia (worm lizards) and Lacertilia 1971). Bisexualism has been described in Leiolepis (lizards). The extant lizards can be further belliana belliana (2n=2x=36), which is widely categorized into five infraorders (the Iguania, found throughout the country, L. belliana ocellata Gekkota, Scincomorpha, Diploglossa, Dibamia, (2n=2x=34) found in upper northern, and L. -

On the Phylogeny and Taxonomy of the Genus Uromastyx Merrem, 1820 (Reptilia: Squamata: Agamidae: Uromastycinae) – Resurrection of the Genus Saara Gray, 1845
Bonner zoologische Beiträge Band 56 (2007) Heft 1/2 Seiten 55–99 Bonn, März 2009 On the Phylogeny and Taxonomy of the Genus Uromastyx Merrem, 1820 (Reptilia: Squamata: Agamidae: Uromastycinae) – Resurrection of the Genus Saara Gray, 1845 Thomas M. WILMS1),4), WOLFGANG BÖHME2), Philipp WAGNER2), Nicolà LUTZMANN2) & Andreas SCHMITZ3) 1)Zoologischer Garten Frankfurt, Bernhard-Grzimek-Allee 1, D-60316 Frankfurt am Main, Germany; E-Mail: [email protected]; 2)Zoologisches Forschungsmuseum A. Koenig, Adenauerallee 160, D- 53113 Bonn, Germany; 3)Muséum d’Histoire naturelle, C. P. 6434, CH-1211 Genève 6, Switzerland; 4)Corresponding author Abstract. We assessed the taxonomic relationships within the genus Uromastyx Merrem, 1820 using morphologi- cal and genetic methods, resulting in the resurrection of the genus Saara Gray, 1845 for Saara hardwickii, S. as- mussi and S. loricata and in changes of the taxonomic rank of Uromastyx nigriventris, U. aegyptia leptieni and U. shobraki. A synopsis of all taxa considered to be valid is provided, including differential diagnosis, description and data on their respective distribution. A key for the species of Saara and Uromastyx is presented. Keywords. Reptilia; Sauria; Agamidae; Uromastycinae; Uromastyx; Saara; Saara hardwickii; Saara asmussi new comb.; Saara loricata new comb.; Uromastyx aegyptia leptieni new status; Uromastyx nigriventris new status; Uromastyx sho- braki new status; Phylogeny; Taxonomy; Morphology. 1. INTRODUCTION Within the Palearctic genus Uromastyx Merrem, 1820 a (KNAPP 2004, WILMS 2007a). But the consumption of total of 17 species are considered to be valid by WILMS spiny-tailed lizards in their countries of origin may be con- & SCHMITZ (2007) and WILMS & BÖHME (2007). -

The Use of Spiny-Tailed Lizards for Medicinal Purposes In
S H O R T C O M M U N I C A T I O N The use of spiny-tailed lizards Uromastyx spp. for medicinal purposes in Peninsular Malaysia Or Oi Ching and Serene C.L. Chng by the required CITES export permits. A study on CITES data trade records of Uromastyx spp. reported over 200 000 INTRODUCTION specimens traded internationally, with an increasing trend DIWHU .QDSS 6SLQ\WDLOHG OL]DUG VSHFLHV DUH piny-tailed lizards Uromastyx spp. consist of 20 also protected by national laws in many range countries. recognized species that inhabit the deserts and Unregulated and unsustainable hunting of spiny-tailed semi-deserts from northern Africa across the lizards may adversely affect the ecosystem (Yom-Tov, Middle East to north-western India (Wilms et al., DVWKH\DUHDQLPSRUWDQWSUH\VSHFLHV &RQVHUYDWLRQ 2009; Wilms, et al., $OVRNQRZQDVGDEERU ,QGLD DQGWKHLUEXUURZVVHUYHDVWKHUPDOUHIXJHV Sdhab lizards, they are hunted and traded for their purported for many other species (Wilms et al., 7KHVHOL]DUGV medicinal value, as well as for meat and for the pet trade feed on plants and insects, providing some degree of pest (Mahmood et al., 2011; Subramanean and Vikram Reddy, control, and are also scavengers (Castilla et al., 2011; 2012; Wilms et al., 2012; Das et al., 2013; Pradhan et al., 6XEUDPDQHDQDQG9LNUDP5HGG\ /DUJHQXPEHUVDUHWDNHQIURPWKHZLOGLQ6DXGL$UDELD DQGVROGWRPLGGOHPHQIRUDURXQG6$5± 86'± BACKGROUND 86' 1 $QRQ )DL]D 3RDFKLQJ WHFKQLTXHV include pouring water or blowing smoke into burrows to The sale of spiny-tailed lizard parts and products used for force -

Acute Respiratory Distress Syndrome in an Uromastix (Uromastyx Acanthinura Nigriventris, 1820)
Author’s Accepted Manuscript Acute Respiratory Distress Syndrome in an Uromastix (Uromastyx acanthinura nigriventris, 1820) Maddalena Iannaccone, Giacomo Rossi, Gian Enrico Magi, Marco Campolo www.sasjournal.com PII: S1557-5063(17)30141-6 DOI: http://dx.doi.org/10.1053/j.jepm.2017.05.007 Reference: JEPM733 To appear in: Journal of Exotic Pet Medicine Cite this article as: Maddalena Iannaccone, Giacomo Rossi, Gian Enrico Magi and Marco Campolo, Acute Respiratory Distress Syndrome in an Uromastix (Uromastyx acanthinura nigriventris, 1820), Journal of Exotic Pet Medicine, http://dx.doi.org/10.1053/j.jepm.2017.05.007 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Case Report Acute Respiratory Distress Syndrome in an Uromastix (Uromastyx acanthinura nigriventris, 1820) Maddalena Iannaccone, DVM Giacomo Rossi, DVM, PhD Gian Enrico Magi, DVM, PhD Marco Campolo, DVM, PhD From the Centro Veterinario Il mondo degli animali esotici, Genova, Italy (Iannaccone), School of Veterinary Medical Sciences, University of Camerino, Matelica, Italy (Rossi, Magi), Centro Veterinario Einaudi, Bari, Italy (Campolo) Address corresponsdence to Maddalena Iannaccone, Centro Veterinario - Il Mondo degli Animali Esotici, Via San Martino 67/r, 16131 Genova, Italy. Email address: [email protected]. Tel: (+39) 010 9820514. -

Zootaxa: a New Polytypic Species of the Genus Uromastyx MERREM 1820
Zootaxa 1394: 1–23 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) A new polytypic species of the genus Uromastyx MERREM 1820 (Reptilia: Squamata: Agamidae: Leiolepidinae)rom f southwestern Arabia THOMAS M. WILMS1,2, 4 & ANDREAS SCHMITZ3 1Reptilium - Terrarien - und Wüstenzoo, Werner-Heisenberg - Str.1, D -76289 Landau, Germany. 2Zoologisches Forschungsmuseum A. Koenig, Adenauerallee 160, D-53113 Bonn, Germany. 3Muséum d'histoire naturelle, C. P. 6434 CH-1211 Genève 6, Switzerland. 4Corresponding author Abstract We describe Uromastyx yemenensis sp. nov. from south-western Arabia, comprising two geographic subspecies, U. y. yemenensis and U. y. shobraki ssp. nov. The new species is a member of the Uromastyx ocellata species group, closely related to U. benti. It is differentiated from its sister taxon by smaller scales around midbody and smaller ventrals. The new species is restricted to the extreme south-western tip of the Arabian Peninsula. The western populations of U. yeme- nensis differ genetically and are constantly distinct in respect to their colour pattern and are therefore recognized as a subspecies. Key words: Reptilia: Sauria: Agamidae: Leiolepidinae: Uromastyx yemenensis sp. nov.; Uromastyx yemenensis sho- braki ssp. nov.; Uromastyx ocellata species group; Uromastyx benti; Uromastyx macfadyeni, Yemen, Arabia Introduction Spiny-tailed lizards of the genus Uromastyx are inhabitants of the desert belt of the Old World, between 5o and 35o N. Their range covers a vast land mass, including northern Africa, Israel, Jordan, Arabia (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, and Yemen), Iran, Iraq, Afghanistan, Pakistan, and north-western India. -

Amphibians and Reptiles of North Africa - Biology, Systematics, Field Guide (1996) 625 Pages, 185 Colourphotographs, Hardcover
AMPHIBIANS AND REPTILES OF Available now ! Jetzt lieferbarNORTH ! AFRICA by H. Hermann Schleich & Werner Kästle, & Klaus Kabisch Schleich, H. H., Kästle, W. & Kabisch, K. Amphibians and Reptiles of North Africa - Biology, Systematics, Field Guide (1996) 625 pages, 185 colourphotographs, Hardcover € 113.- / US$ 126,- ISBN 3-87429-377-7 (060987) The lack of a comprehensive work on the herpetofauna of North Africa has remained a handicap to our days, in spite of the fact that reptiles play a key role in desert ecosystems and show a surprising wealth of forms in the Mediterranean zone. This book tries to fill this gap as far as the Maghreb (Morocco, Algeria, Tunisia) and Libya is concerned. In the introductory chapters much space has been devoted to characterize the wide span of ambiental conditions from dense forest to scorched desert. Synoptic topics cover different aspects as biotopes, activity patterns, physiological adaptations, food chains and webs, communities of amphibians and reptiles, or zoogeographic aspects. In a series of north-south-transsects through the countries of the region hundreds of data on the local distribution of species are summed up and give an overview of the regional herpetofaunas. The special chapters which cover most of the contens treat the species (there are about 100) separately and emphasize their biology, devoting much room to ecology, behaviour and reproduction. The authors evaluated data from hundreds of scientific works, but the contents also reflect their own work, presenting unpublished field data considering thermal behaviour, fecal pellet analyses and observations on behaviour. Social interrelations are presented in a series of new flow diagrams. -

Uromastyx Acanthinura Bell, 1825
AC22 Doc. 10.2 Annex 6a Uromastyx acanthinura Bell, 1825 FAMILY: Agamidae COMMON NAMES: Bell's Dabb Lizard, Black Spiny-tailed Lizard, Dabb's Mastigure (English); Dob, Fouette-queue Epineux (French); Lagarto de Cola Espinosa Común (Spanish). GLOBAL CONSERVATION STATUS: Accepted for inclusion in the 2006 IUCN Red List of Threatened Species (IUCNa, in prep.) as Near Threatened. Listing is based on a significant decline due to unsustainable harvest for food, medicine and the international pet trade, and because its habitat is being degraded, making the species close to qualifying for Vulnerable under Criteria A2cd (IUCNb, in prep.). SIGNIFICANT TRADE REVIEW FOR: Algeria, Libyan Arab Jamahiriya (LAJ) Range States selected for review Range State Exports* Urgent, possible Comments (1994-2003) or least concern Algeria 0 Least concern Small quantities (total 92 specimens) reported by Spain as imported from Algeria, country of origin recorded as unknown Libyan Arab 20 Least concern Minimal reported trade. Jamahiriya *Excluding re-exports. SUMMARY Uromastyx acanthinura, often known as the North African Spiny-tailed Lizard, is a medium–sized lizard occurring in desert habitats of north-western Africa, with records from the northern part of western Libyan Arab Jamahiriya, Tunisia, the northern half of Algeria, Morocco and the northern part of Western Sahara. All species of Uromastyx were listed in CITES Appendix II in 1977. Uromastyx species are traded internationally for the pet trade. The level of trade is variable between the species. For many years U. acanthinura was the dominant Uromastyx species in trade. The USA has been the main reported importer of U. acanthinura, with considerable numbers also going to Europe and Japan. -

The First Iguanian Lizard from the Mesozoic of Africa Rsos.Royalsocietypublishing.Org Sebastián Apesteguía1,Juand.Daza2, Tiago R
Downloaded from http://rsos.royalsocietypublishing.org/ on October 3, 2016 The first iguanian lizard from the Mesozoic of Africa rsos.royalsocietypublishing.org Sebastián Apesteguía1,JuanD.Daza2, Tiago R. Simões3 and Jean Claude Rage4 Research 1CEBBAD (CONICET), Fundación de Historia Natural ‘Félix de Azara’, Universidad Maimónides, Hidalgo 775, 7°p (1405), Buenos Aires, Argentina Cite this article: Apesteguía S, Daza JD, 2Department of Biological Sciences, Sam Houston State University, 1900 Avenue I Lee Simões TR, Rage JC. 2016 The first iguanian Drain Building Suite 300, Huntsville, TX 77341-2116, USA lizard from the Mesozoic of Africa. R. Soc. open 3Department of Biological Sciences, University of Alberta, Edmonton, Alberta, sci. 3: 160462. Canada T6G2E9 http://dx.doi.org/10.1098/rsos.160462 4CR2P,Sorbonne Universités, UMR 7207 CNRS, CNRS, Muséum National d’Histoire Naturelle, Université Paris 6, CP 38, rue Cuvier, 75231, Paris cedex 05, France SA, 0000-0002-0414-0524 Received: 28 June 2016 Accepted: 22 August 2016 The fossil record shows that iguanian lizards were widely distributed during the Late Cretaceous. However, the biogeographic history and early evolution of one of its most diverse and peculiar clades (acrodontans) remain poorly Subject Category: known. Here, we present the first Mesozoic acrodontan from Africa, which also represents the oldest iguanian lizard from Earth science that continent. The new taxon comes from the Kem Kem Beds in Morocco (Cenomanian, Late Cretaceous) and is based Subject Areas: on a partial lower jaw. The new taxon presents a number of palaeontology/taxonomy and systematics features that are found only among acrodontan lizards and shares greatest similarities with uromastycines, specifically.