SGK2 Active Human (S7570)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Epithelial Ovarian Cancer: Searching for New Modulators of Drug Resistance
UNIVERSITÀ DEGLI STUDI DI UDINE Dipartimento di scienze mediche e biologiche PhD COURSE IN BIOMEDICAL SCIENCES AND BIOTECHNOLOGY XXIX cycle PhD THESIS Epithelial Ovarian Cancer: searching for new modulators of drug resistance. PhD student Supervisor Prof. Carlo Ennio Michele Pucillo Valentina Ranzuglia Co-supervisors Dr. Gustavo Baldassarre Dr. Monica Schiappacassi PhD coordinator Prof. Claudio Brancolini Academic year 2015/2016 This PhD work was done at Centro di Riferimento Oncologico (CRO, National Cancer Institute ) of Aviano, in the Division of Experimental Oncology 2 directed by Dr. Gustavo Baldassarre. i Table of contents Abstract 3 1. Introduction 4 1.1 Epithelial Ovarian Cancer 4 1.1.1 Platinum resistance 5 1.2 Functional Genomic Screening 8 1.3 Serum and glucocorticoid-regulated kinases 9 1.3.1 SGK structure 10 1.3.2 SGK activation and regulation 11 1.3.3 Role of SGKs in regulation of molecular and cellular functions 13 1.3.4 Serum- and glucocorticoid-regulated kinases in cancer 15 1.4 Autophagy 17 2.Aim of the study 21 3.Results 22 3.1 Identification of SGK2 as a mediator of platinum sensitivity in EOC cells. 22 3.2 SGK2 silencing sensitizes EOC cells to platinum. 23 3.3 SGK2 overexpression confers an increased resistance to platinum drug. 25 3.4 SGK2-overexpressing cells present an increased in vitro and in vivo growth 26 rate 3.5 SGK2 kinase activity is involved in EOC sensitivity to platinum treatment. 28 3.6 The SGK1/SGK2 kinase inhibitor, GSK650394, reduces cell viability in 29 combination with platinum. 3.7 GSK650394 is able to make SGK2-expressing cells more sensitive to platinum. -

AGC Kinases in Mtor Signaling, in Mike Hall and Fuyuhiko Tamanoi: the Enzymes, Vol
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book, The Enzymes, Vol .27, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: ESTELA JACINTO, AGC Kinases in mTOR Signaling, In Mike Hall and Fuyuhiko Tamanoi: The Enzymes, Vol. 27, Burlington: Academic Press, 2010, pp.101-128. ISBN: 978-0-12-381539-2, © Copyright 2010 Elsevier Inc, Academic Press. Author's personal copy 7 AGC Kinases in mTOR Signaling ESTELA JACINTO Department of Physiology and Biophysics UMDNJ-Robert Wood Johnson Medical School, Piscataway New Jersey, USA I. Abstract The mammalian target of rapamycin (mTOR), a protein kinase with homology to lipid kinases, orchestrates cellular responses to growth and stress signals. Various extracellular and intracellular inputs to mTOR are known. mTOR processes these inputs as part of two mTOR protein com- plexes, mTORC1 or mTORC2. Surprisingly, despite the many cellular functions that are linked to mTOR, there are very few direct mTOR substrates identified to date. -
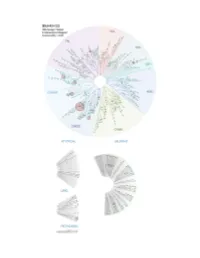
Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) BSJ-03-123 AAK1 AAK1 94 1000 BSJ-03-123 ABL1(E255K)-phosphorylated ABL1 79 1000 BSJ-03-123 ABL1(F317I)-nonphosphorylated ABL1 89 1000 BSJ-03-123 ABL1(F317I)-phosphorylated ABL1 98 1000 BSJ-03-123 ABL1(F317L)-nonphosphorylated ABL1 86 1000 BSJ-03-123 ABL1(F317L)-phosphorylated ABL1 89 1000 BSJ-03-123 ABL1(H396P)-nonphosphorylated ABL1 76 1000 BSJ-03-123 ABL1(H396P)-phosphorylated ABL1 90 1000 BSJ-03-123 ABL1(M351T)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1(Q252H)-nonphosphorylated ABL1 56 1000 BSJ-03-123 ABL1(Q252H)-phosphorylated ABL1 97 1000 BSJ-03-123 ABL1(T315I)-nonphosphorylated ABL1 100 1000 BSJ-03-123 ABL1(T315I)-phosphorylated ABL1 85 1000 BSJ-03-123 ABL1(Y253F)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1-nonphosphorylated ABL1 60 1000 BSJ-03-123 ABL1-phosphorylated ABL1 79 1000 BSJ-03-123 ABL2 ABL2 89 1000 BSJ-03-123 ACVR1 ACVR1 100 1000 BSJ-03-123 ACVR1B ACVR1B 95 1000 BSJ-03-123 ACVR2A ACVR2A 100 1000 BSJ-03-123 ACVR2B ACVR2B 96 1000 BSJ-03-123 ACVRL1 ACVRL1 84 1000 BSJ-03-123 ADCK3 CABC1 90 1000 BSJ-03-123 ADCK4 ADCK4 91 1000 BSJ-03-123 AKT1 AKT1 100 1000 BSJ-03-123 AKT2 AKT2 98 1000 BSJ-03-123 AKT3 AKT3 100 1000 BSJ-03-123 ALK ALK 100 1000 BSJ-03-123 ALK(C1156Y) ALK 78 1000 BSJ-03-123 ALK(L1196M) ALK 100 1000 BSJ-03-123 AMPK-alpha1 PRKAA1 93 1000 BSJ-03-123 AMPK-alpha2 PRKAA2 100 1000 BSJ-03-123 ANKK1 ANKK1 89 1000 BSJ-03-123 ARK5 NUAK1 98 1000 BSJ-03-123 ASK1 MAP3K5 100 1000 BSJ-03-123 ASK2 MAP3K6 92 1000 BSJ-03-123 AURKA -

SGK3 Sirna Set I Sirna Duplexes Targeted Against Three Exon Regions
Catalog # Aliquot Size S08-911-05 3 x 5 nmol S08-911-20 3 x 20 nmol S08-911-50 3 x 50 nmol SGK3 siRNA Set I siRNA duplexes targeted against three exon regions Catalog # S08-911 Lot # Z2085-77 Specificity Formulation SGK3 siRNAs are designed to specifically knock-down The siRNAs are supplied as a lyophilized powder and human SGK3 expression. shipped at room temperature. Product Description Reconstitution Protocol SGK3 siRNA is a pool of three individual synthetic siRNA Briefly centrifuge the tubes (maximum RCF 4,000g) to duplexes designed to knock-down human SGK3 mRNA collect lyophilized siRNA at the bottom of the tube. expression. Each siRNA is 19-25 bases in length. The gene Resuspend the siRNA in 50 µl of DEPC-treated water accession number is NM_013257. (supplied by researcher), which results in a 1x stock solution (10 µM). Gently pipet the solution 3-5 times to mix Gene Aliases and avoid the introduction of bubbles. Optional: aliquot CISK, SGKL 1x stock solutions for storage. Storage and Stability Related Products The lyophilized powder is stable for at least 4 weeks at room temperature. It is recommended that the Product Name Catalog Number lyophilized and resuspended siRNAs are stored at or SGK1, Active S06-11G below -20oC. After resuspension, siRNA stock solutions ≥2 SGK2, Active S07-10G µM can undergo up to 50 freeze-thaw cycles without SGK3, Active S08-10G significant degradation. For long-term storage, it is SGK1, Unactive S06-15G recommended that the siRNA is stored at -70oC. For most SGK2, Unactive S07-14G favorable performance, avoid repeated handling and SGK3, Unactive S08-14G multiple freeze/thaw cycles. -

Kinase Profiling Book
Custom and Pre-Selected Kinase Prof iling to f it your Budget and Needs! As of July 1, 2021 19.8653 mm 128 196 12 Tyrosine Serine/Threonine Lipid Kinases Kinases Kinases Carna Biosciences, Inc. 2007 Carna Biosciences, Inc. Profiling Assays available from Carna Biosciences, Inc. As of July 1, 2021 Page Kinase Name Assay Platform Page Kinase Name Assay Platform 4 ABL(ABL1) MSA 21 EGFR[T790M/C797S/L858R] MSA 4 ABL(ABL1)[E255K] MSA 21 EGFR[T790M/L858R] MSA 4 ABL(ABL1)[T315I] MSA 21 EPHA1 MSA 4 ACK(TNK2) MSA 21 EPHA2 MSA 4 AKT1 MSA 21 EPHA3 MSA 5 AKT2 MSA 22 EPHA4 MSA 5 AKT3 MSA 22 EPHA5 MSA 5 ALK MSA 22 EPHA6 MSA 5 ALK[C1156Y] MSA 22 EPHA7 MSA 5 ALK[F1174L] MSA 22 EPHA8 MSA 6 ALK[G1202R] MSA 23 EPHB1 MSA 6 ALK[G1269A] MSA 23 EPHB2 MSA 6 ALK[L1196M] MSA 23 EPHB3 MSA 6 ALK[R1275Q] MSA 23 EPHB4 MSA 6 ALK[T1151_L1152insT] MSA 23 Erk1(MAPK3) MSA 7 EML4-ALK MSA 24 Erk2(MAPK1) MSA 7 NPM1-ALK MSA 24 Erk5(MAPK7) MSA 7 AMPKα1/β1/γ1(PRKAA1/B1/G1) MSA 24 FAK(PTK2) MSA 7 AMPKα2/β1/γ1(PRKAA2/B1/G1) MSA 24 FER MSA 7 ARG(ABL2) MSA 24 FES MSA 8 AurA(AURKA) MSA 25 FGFR1 MSA 8 AurA(AURKA)/TPX2 MSA 25 FGFR1[V561M] MSA 8 AurB(AURKB)/INCENP MSA 25 FGFR2 MSA 8 AurC(AURKC) MSA 25 FGFR2[V564I] MSA 8 AXL MSA 25 FGFR3 MSA 9 BLK MSA 26 FGFR3[K650E] MSA 9 BMX MSA 26 FGFR3[K650M] MSA 9 BRK(PTK6) MSA 26 FGFR3[V555L] MSA 9 BRSK1 MSA 26 FGFR3[V555M] MSA 9 BRSK2 MSA 26 FGFR4 MSA 10 BTK MSA 27 FGFR4[N535K] MSA 10 BTK[C481S] MSA 27 FGFR4[V550E] MSA 10 BUB1/BUB3 MSA 27 FGFR4[V550L] MSA 10 CaMK1α(CAMK1) MSA 27 FGR MSA 10 CaMK1δ(CAMK1D) MSA 27 FLT1 MSA 11 CaMK2α(CAMK2A) MSA 28 -

Supplementary Table 1. in Vitro Side Effect Profiling Study for LDN/OSU-0212320. Neurotransmitter Related Steroids
Supplementary Table 1. In vitro side effect profiling study for LDN/OSU-0212320. Percent Inhibition Receptor 10 µM Neurotransmitter Related Adenosine, Non-selective 7.29% Adrenergic, Alpha 1, Non-selective 24.98% Adrenergic, Alpha 2, Non-selective 27.18% Adrenergic, Beta, Non-selective -20.94% Dopamine Transporter 8.69% Dopamine, D1 (h) 8.48% Dopamine, D2s (h) 4.06% GABA A, Agonist Site -16.15% GABA A, BDZ, alpha 1 site 12.73% GABA-B 13.60% Glutamate, AMPA Site (Ionotropic) 12.06% Glutamate, Kainate Site (Ionotropic) -1.03% Glutamate, NMDA Agonist Site (Ionotropic) 0.12% Glutamate, NMDA, Glycine (Stry-insens Site) 9.84% (Ionotropic) Glycine, Strychnine-sensitive 0.99% Histamine, H1 -5.54% Histamine, H2 16.54% Histamine, H3 4.80% Melatonin, Non-selective -5.54% Muscarinic, M1 (hr) -1.88% Muscarinic, M2 (h) 0.82% Muscarinic, Non-selective, Central 29.04% Muscarinic, Non-selective, Peripheral 0.29% Nicotinic, Neuronal (-BnTx insensitive) 7.85% Norepinephrine Transporter 2.87% Opioid, Non-selective -0.09% Opioid, Orphanin, ORL1 (h) 11.55% Serotonin Transporter -3.02% Serotonin, Non-selective 26.33% Sigma, Non-Selective 10.19% Steroids Estrogen 11.16% 1 Percent Inhibition Receptor 10 µM Testosterone (cytosolic) (h) 12.50% Ion Channels Calcium Channel, Type L (Dihydropyridine Site) 43.18% Calcium Channel, Type N 4.15% Potassium Channel, ATP-Sensitive -4.05% Potassium Channel, Ca2+ Act., VI 17.80% Potassium Channel, I(Kr) (hERG) (h) -6.44% Sodium, Site 2 -0.39% Second Messengers Nitric Oxide, NOS (Neuronal-Binding) -17.09% Prostaglandins Leukotriene, -

Protein Kinase C As a Paradigm Alexandra C
Biochem. J. (2003) 370, 361–371 (Printed in Great Britain) 361 REVIEW ARTICLE Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm Alexandra C. NEWTON1 Department of Pharmacology, University of California at San Diego, La Jolla, CA 92093-0640, U.S.A. Phosphorylation plays a central role in regulating the activation down-regulation of PKC as a paradigm for how these sites and signalling lifetime of protein kinases A, B (also known as control the function of the ABC kinases. Akt) and C. These kinases share three conserved phosphorylation motifs: the activation loop segment, the turn motif and the Key words: phosphoinositide 3-kinase, phosphoinositide-depen- hydrophobic motif. This review focuses on how phosphorylation dent kinase-1 (PDK-1), phosphorylation motif, protein kinase A at each of these sites regulates the maturation, signalling and (PKA), protein kinase B (PKB)\Akt, protein kinase C (PKC). INTRODUCTION by phosphorylation as a paradigm for control of ABC kinase function by phosphorylation. Almost half a century ago Krebs, Graves and Fischer reported b that the first discovered protein kinase, phosphorylase kinase, ARCHITECTURE OF ABC KINASES was, itself, activated by phosphorylation [1]. Two decades later, Fischer and colleagues showed that the kinase that phosphory- Protein kinases A, B\Akt and C have in common a conserved lates phosphorylase kinase, later named protein kinase A (PKA), kinase core whose function is regulated allosterically by a was also phosphorylated [2]. Reversible control by phosphory- corresponding regulatory moiety (Figure 1). In the case of PKA, lation\dephosphorylation is now firmly established as a fun- the regulatory moiety is a separate polypeptide, whereas the damental mechanism for regulating the function of most of the regulatory determinants are on the same polypeptide as the 500 or so members of the mammalian protein kinase superfamily. -

And Glucocorticoid-Inducible Kinase (Sgk1)" (2007)
Dartmouth College Dartmouth Digital Commons Open Dartmouth: Published works by Dartmouth faculty Faculty Work 2-14-2007 Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid- Inducible Kinase (Sgk1) J. Denry Sato Mount Desert Island Biological Laboratory, Salisbury Cove M. Christine Chapline Dartmouth College Renee Thibodeau Dartmouth College Raymond A. Frizzell University of Pittsburgh Bruce A. Stanton Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Medicine and Health Sciences Commons Dartmouth Digital Commons Citation Sato, J. Denry; Chapline, M. Christine; Thibodeau, Renee; Frizzell, Raymond A.; and Stanton, Bruce A., "Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid-Inducible Kinase (Sgk1)" (2007). Open Dartmouth: Published works by Dartmouth faculty. 3156. https://digitalcommons.dartmouth.edu/facoa/3156 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Open Dartmouth: Published works by Dartmouth faculty by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. Original Paper Cellular Physiology Cell Physiol Biochem 2007;20:91-98 Accepted: February 14, 2007 and Biochemistry Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by Serum- and Glucocorticoid-Inducible Kinase (SGK1) J. Denry Sato1, M. Christine Chapline1,2, Renee Thibodeau1,2, Raymond A. Frizzell1,3 and Bruce A. Stanton1,2 1Mount Desert Island Biological Laboratory, Salisbury Cove, 2Dept. of Physiology, Dartmouth Medical School, Hanover, 3Dept. of Cell Biology and Physiology, University of Pittsburgh Medical School, Pittsburgh Key Words in stimulating CFTR Cl currents. -

An Evolutionary-Conserved Redox Regulatory Mechanism in Human Ser/Thr Protein Kinases
bioRxiv preprint doi: https://doi.org/10.1101/571844; this version posted March 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. An evolutionary-conserved redox regulatory mechanism in human Ser/Thr protein kinases Dominic P. Byrne1*, Safal Shrestha2,3, Natarajan Kannan2.3 and Patrick A. Eyers1* 1 Department of Biochemistry, Institute of Integrative Biology, University of Liverpool, Liverpool, L69 7ZB, UK 2 Institute of Bioinformatics, University of Georgia, Athens, GA 30602, USA 3 Department of Biochemistry & Molecular Biology, University of Georgia, Athens, GA 30602, USA * Correspondence to [email protected] or [email protected] ONE-SENTENCE SUMMARY: The catalytic activity of Ser/Thr kinases is regulated through a conserved Cys-based redox mechanism. ABSTRACT: Reactive oxygen species (ROS) are products of oxygen metabolism, but are also recognized as endogenous physiological mediators of cellular signaling. Eukaryotic protein kinase (ePK) regulation occurs through reversible phosphorylation events in the flexible activation segment. In this study, we demonstrate that the catalytic phosphotransferase output from the mitotic Ser/Thr kinase Aurora A is also controlled by cysteine (Cys) oxidation. Reversible regulation occurs by direct modification of a conserved residue (Cys 290), which lies adjacent to Thr 288, the activating site of phosphorylation. Strikingly, redox modulation of the Cys 290-equivalent in other ePKs is predicted to be an underappreciated regulatory mechanism, since ~100 human Ser/Thr kinases possess a Cys at this position in the conserved activation loop. -

Combining Micro-RNA and Protein Sequencing to Detect Robust
www.nature.com/scientificreports OPEN Combining micro-RNA and protein sequencing to detect robust biomarkers for Graves’ disease and Received: 2 March 2018 Accepted: 15 May 2018 orbitopathy Published: xx xx xxxx Lei Zhang1, Giulia Masetti1,2, Giuseppe Colucci3, Mario Salvi 3, Danila Covelli3, Anja Eckstein4, Ulrike Kaiser4, Mohd Shazli Draman 1, Ilaria Muller 1, Marian Ludgate1, Luigi Lucini 5 & Filippo Biscarini 1,6 Graves’ Disease (GD) is an autoimmune condition in which thyroid-stimulating antibodies (TRAB) mimic thyroid-stimulating hormone function causing hyperthyroidism. 5% of GD patients develop infammatory Graves’ orbitopathy (GO) characterized by proptosis and attendant sight problems. A major challenge is to identify which GD patients are most likely to develop GO and has relied on TRAB measurement. We screened sera/plasma from 14 GD, 19 GO and 13 healthy controls using high-throughput proteomics and miRNA sequencing (Illumina’s HiSeq2000 and Agilent-6550 Funnel quadrupole-time-of-fight mass spectrometry) to identify potential biomarkers for diagnosis or prognosis evaluation. Euclidean distances and diferential expression (DE) based on miRNA and protein quantifcation were analysed by multidimensional scaling (MDS) and multinomial regression respectively. We detected 3025 miRNAs and 1886 proteins and MDS revealed good separation of the 3 groups. Biomarkers were identifed by combined DE and Lasso-penalized predictive models; accuracy of predictions was 0.86 (±0:18), and 5 miRNA and 20 proteins were found including Zonulin, Alpha-2 macroglobulin, Beta-2 glycoprotein 1 and Fibronectin. Functional analysis identifed relevant metabolic pathways, including hippo signaling, bacterial invasion of epithelial cells and mRNA surveillance. Proteomic and miRNA analyses, combined with robust bioinformatics, identifed circulating biomarkers applicable to diagnose GD, predict GO disease status and optimize patient management. -

SGK2, Active Full-Length Recombinant Protein Expressed in Sf9 Cells
570-5600 Parkwood Way Richmond, BC V6V2M2 CANADA Tel: 1-(604)-232-4600 Fax: 1-(604)-232-4601 [email protected] SGK2, Active Full-length recombinant protein expressed in Sf9 cells Catalog # S07-10G Lot # C162-2 Product Description Specific Activity Recombinant full-length human SGK2 was expressed by baculovirus in Sf9 insect cells using an N-terminal GST tag. 660,000 The gene accession number is NM_170693. 495,000 330,000 Gene Aliases 165,000 H-SGK2; dJ138B7.2 Activity (cpm) 0 0 80 160 240 320 Formulation Protein (ng) The specific activity of SGK2 was determined to be 94 nmol Recombinant protein stored in 50mM Tris-HCl, pH 7.5, /min/mg as per activity assay protocol. 150mM NaCl, 0.25mM DTT, 0.1mM EGTA, 0.1mM EDTA, Purity 0.1mM PMSF, 25% glycerol. Storage and Stability Store product at –70oC. For optimal storage, aliquot target into smaller quantities after centrifugation and The purity was determined to be store at recommended temperature. For most favorable >90% by densitometry. performance, avoid repeated handling and multiple Approx. MW 71kDa. freeze/thaw cycles. Scientific Background SGK2 is a member of the serum- and glucocorticoid- SGK2, Active induced kinases (SGK) which are serine-threonine kinases Full-length recombinant protein expressed in Sf9 cells and belong to the "AGC" kinase subfamily, which includes protein kinases A, G, and C, and its catalytic Catalog Number S07-10G domain is most similar to protein kinase B (PKB) (1). SGK2, Specific Activity 94 nmol/min/mg like the other two isoforms SGK1 and SGK3, is stimulated Specific Lot Number C162-2 by insulin and insulin-like growth factor-1 (IGF-1), and has Purity >90% been shown to enhance Na(+)/K(+)-ATPase activity in a Concentration 0.1µg/µl variety of cells (2). -

Mtor Signaling in Metabolism and Cancer
cells Editorial mTOR Signaling in Metabolism and Cancer Shile Huang 1,2 1 Department of Biochemistry and Molecular Biology, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130-3932, USA; [email protected]; Tel.: +1-318-675-7759 2 Feist-Weiller Cancer Center, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130-3932, USA Received: 10 October 2020; Accepted: 13 October 2020; Published: 13 October 2020 Abstract: The mechanistic/mammalian target of rapamycin (mTOR), a serine/threonine kinase, is a central regulator for human physiological activity. Deregulated mTOR signaling is implicated in a variety of disorders, such as cancer, obesity, diabetes, and neurodegenerative diseases. The papers published in this special issue summarize the current understanding of the mTOR pathway and its role in the regulation of tissue regeneration, regulatory T cell differentiation and function, and different types of cancer including hematologic malignancies, skin, prostate, breast, and head and neck cancer. The findings highlight that targeting the mTOR pathway is a promising strategy to fight against certain human diseases. Keywords: mTOR; PI3K; Akt; tissue regeneration; regulatory T cells; tumor; photodynamic therapy The mechanistic/mammalian target of rapamycin (mTOR), a serine/threonine kinase, integrates environmental cues such as hormones, growth factors, nutrients, oxygen, and energy, regulating cell growth, proliferation, survival, motility and differentiation as well as metabolism (reviewed in [1,2]). Evidence has demonstrated that deregulated mTOR signaling is implicated in a variety of disorders, such as cancer, obesity, diabetes, and neurodegenerative diseases (reviewed in [1,2]). Current knowledge indicates that mTOR functions at least as two distinct complexes (mTORC1 and mTORC2) in mammalian cells.