Mtor Signaling in Metabolism and Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AGC Kinases in Mtor Signaling, in Mike Hall and Fuyuhiko Tamanoi: the Enzymes, Vol
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book, The Enzymes, Vol .27, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: ESTELA JACINTO, AGC Kinases in mTOR Signaling, In Mike Hall and Fuyuhiko Tamanoi: The Enzymes, Vol. 27, Burlington: Academic Press, 2010, pp.101-128. ISBN: 978-0-12-381539-2, © Copyright 2010 Elsevier Inc, Academic Press. Author's personal copy 7 AGC Kinases in mTOR Signaling ESTELA JACINTO Department of Physiology and Biophysics UMDNJ-Robert Wood Johnson Medical School, Piscataway New Jersey, USA I. Abstract The mammalian target of rapamycin (mTOR), a protein kinase with homology to lipid kinases, orchestrates cellular responses to growth and stress signals. Various extracellular and intracellular inputs to mTOR are known. mTOR processes these inputs as part of two mTOR protein com- plexes, mTORC1 or mTORC2. Surprisingly, despite the many cellular functions that are linked to mTOR, there are very few direct mTOR substrates identified to date. -

Phosphatidylinositol-3-Kinase Related Kinases (Pikks) in Radiation-Induced Dna Damage
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2012, vol. 81(4), p. 177-187 ISSN 0372-7025 DOI: 10.31482/mmsl.2012.025 REVIEW ARTICLE PHOSPHATIDYLINOSITOL-3-KINASE RELATED KINASES (PIKKS) IN RADIATION-INDUCED DNA DAMAGE Ales Tichy 1, Kamila Durisova 1, Eva Novotna 1, Lenka Zarybnicka 1, Jirina Vavrova 1, Jaroslav Pejchal 2, Zuzana Sinkorova 1 1 Department of Radiobiology, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic 2 Centrum of Advanced Studies, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic. Received 5 th September 2012. Revised 27 th November 2012. Published 7 th December 2012. Summary This review describes a drug target for cancer therapy, family of phosphatidylinositol-3 kinase related kinases (PIKKs), and it gives a comprehensive review of recent information. Besides general information about phosphatidylinositol-3 kinase superfamily, it characterizes a DNA-damage response pathway since it is monitored by PIKKs. Key words: PIKKs; ATM; ATR; DNA-PK; Ionising radiation; DNA-repair ABBREVIATIONS therapy and radiation play a pivotal role. Since cancer is one of the leading causes of death worldwide, it is DSB - double stand breaks, reasonable to invest time and resources in the enligh - IR - ionising radiation, tening of mechanisms, which underlie radio-resis - p53 - TP53 tumour suppressors, tance. PI - phosphatidylinositol. The aim of this review is to describe the family INTRODUCTION of phosphatidyinositol 3-kinases (PI3K) and its func - tional subgroup - phosphatidylinositol-3-kinase rela - An efficient cancer treatment means to restore ted kinases (PIKKs) and their relation to repairing of controlled tissue growth via interfering with cell sig - radiation-induced DNA damage. -
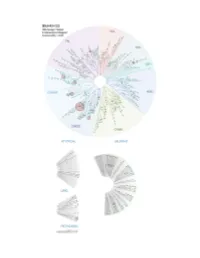
Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) BSJ-03-123 AAK1 AAK1 94 1000 BSJ-03-123 ABL1(E255K)-phosphorylated ABL1 79 1000 BSJ-03-123 ABL1(F317I)-nonphosphorylated ABL1 89 1000 BSJ-03-123 ABL1(F317I)-phosphorylated ABL1 98 1000 BSJ-03-123 ABL1(F317L)-nonphosphorylated ABL1 86 1000 BSJ-03-123 ABL1(F317L)-phosphorylated ABL1 89 1000 BSJ-03-123 ABL1(H396P)-nonphosphorylated ABL1 76 1000 BSJ-03-123 ABL1(H396P)-phosphorylated ABL1 90 1000 BSJ-03-123 ABL1(M351T)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1(Q252H)-nonphosphorylated ABL1 56 1000 BSJ-03-123 ABL1(Q252H)-phosphorylated ABL1 97 1000 BSJ-03-123 ABL1(T315I)-nonphosphorylated ABL1 100 1000 BSJ-03-123 ABL1(T315I)-phosphorylated ABL1 85 1000 BSJ-03-123 ABL1(Y253F)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1-nonphosphorylated ABL1 60 1000 BSJ-03-123 ABL1-phosphorylated ABL1 79 1000 BSJ-03-123 ABL2 ABL2 89 1000 BSJ-03-123 ACVR1 ACVR1 100 1000 BSJ-03-123 ACVR1B ACVR1B 95 1000 BSJ-03-123 ACVR2A ACVR2A 100 1000 BSJ-03-123 ACVR2B ACVR2B 96 1000 BSJ-03-123 ACVRL1 ACVRL1 84 1000 BSJ-03-123 ADCK3 CABC1 90 1000 BSJ-03-123 ADCK4 ADCK4 91 1000 BSJ-03-123 AKT1 AKT1 100 1000 BSJ-03-123 AKT2 AKT2 98 1000 BSJ-03-123 AKT3 AKT3 100 1000 BSJ-03-123 ALK ALK 100 1000 BSJ-03-123 ALK(C1156Y) ALK 78 1000 BSJ-03-123 ALK(L1196M) ALK 100 1000 BSJ-03-123 AMPK-alpha1 PRKAA1 93 1000 BSJ-03-123 AMPK-alpha2 PRKAA2 100 1000 BSJ-03-123 ANKK1 ANKK1 89 1000 BSJ-03-123 ARK5 NUAK1 98 1000 BSJ-03-123 ASK1 MAP3K5 100 1000 BSJ-03-123 ASK2 MAP3K6 92 1000 BSJ-03-123 AURKA -

Diverse Mechanisms Activate the PI 3-Kinase/Mtor Pathway In
Tran et al. BMC Cancer (2021) 21:136 https://doi.org/10.1186/s12885-021-07826-4 RESEARCH ARTICLE Open Access Diverse mechanisms activate the PI 3- kinase/mTOR pathway in melanomas: implications for the use of PI 3-kinase inhibitors to overcome resistance to inhibitors of BRAF and MEK Khanh B. Tran1,2,3, Sharada Kolekar1, Anower Jabed2, Patrick Jaynes2, Jen-Hsing Shih2, Qian Wang2, Jack U. Flanagan1,3, Gordon W. Rewcastle1,3, Bruce C. Baguley1,3 and Peter R. Shepherd1,2,3* Abstract Background: The PI 3-kinase (PI3K) pathway has been implicated as a target for melanoma therapy. Methods: Given the high degree of genetic heterogeneity in melanoma, we sought to understand the breadth of variation in PI3K signalling in the large NZM panel of early passage cell lines developed from metastatic melanomas. Results: We find the vast majority of lines show upregulation of this pathway, and this upregulation is achieved by a wide range of mechanisms. Expression of all class-IA PI3K isoforms was readily detected in these cell lines. A range of genetic changes in different components of the PI3K pathway was seen in different lines. Coding variants or amplification were identified in the PIK3CA gene, and amplification of the PK3CG gene was common. Deletions in the PIK3R1 and PIK3R2 regulatory subunits were also relatively common. Notably, no genetic variants were seen in the PIK3CD gene despite p110δ being expressed in many of the lines. Genetic variants were detected in a number of genes that encode phosphatases regulating the PI3K signalling, with reductions in copy number common in PTEN, INPP4B, INPP5J, PHLLP1 and PHLLP2 genes. -

Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase
cells Review Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase Jaroslav Kalous *, Denisa Jansová and Andrej Šušor Institute of Animal Physiology and Genetics, Academy of Sciences of the Czech Republic, Rumburska 89, 27721 Libechov, Czech Republic; [email protected] (D.J.); [email protected] (A.Š.) * Correspondence: [email protected] Received: 28 April 2020; Accepted: 24 June 2020; Published: 27 June 2020 Abstract: Cyclin dependent kinase 1 (CDK1) has been primarily identified as a key cell cycle regulator in both mitosis and meiosis. Recently, an extramitotic function of CDK1 emerged when evidence was found that CDK1 is involved in many cellular events that are essential for cell proliferation and survival. In this review we summarize the involvement of CDK1 in the initiation and elongation steps of protein synthesis in the cell. During its activation, CDK1 influences the initiation of protein synthesis, promotes the activity of specific translational initiation factors and affects the functioning of a subset of elongation factors. Our review provides insights into gene expression regulation during the transcriptionally silent M-phase and describes quantitative and qualitative translational changes based on the extramitotic role of the cell cycle master regulator CDK1 to optimize temporal synthesis of proteins to sustain the division-related processes: mitosis and cytokinesis. Keywords: CDK1; 4E-BP1; mTOR; mRNA; translation; M-phase 1. Introduction 1.1. Cyclin Dependent Kinase 1 (CDK1) Is a Subunit of the M Phase-Promoting Factor (MPF) CDK1, a serine/threonine kinase, is a catalytic subunit of the M phase-promoting factor (MPF) complex which is essential for cell cycle control during the G1-S and G2-M phase transitions of eukaryotic cells. -

Protein Kinase C As a Paradigm Alexandra C
Biochem. J. (2003) 370, 361–371 (Printed in Great Britain) 361 REVIEW ARTICLE Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm Alexandra C. NEWTON1 Department of Pharmacology, University of California at San Diego, La Jolla, CA 92093-0640, U.S.A. Phosphorylation plays a central role in regulating the activation down-regulation of PKC as a paradigm for how these sites and signalling lifetime of protein kinases A, B (also known as control the function of the ABC kinases. Akt) and C. These kinases share three conserved phosphorylation motifs: the activation loop segment, the turn motif and the Key words: phosphoinositide 3-kinase, phosphoinositide-depen- hydrophobic motif. This review focuses on how phosphorylation dent kinase-1 (PDK-1), phosphorylation motif, protein kinase A at each of these sites regulates the maturation, signalling and (PKA), protein kinase B (PKB)\Akt, protein kinase C (PKC). INTRODUCTION by phosphorylation as a paradigm for control of ABC kinase function by phosphorylation. Almost half a century ago Krebs, Graves and Fischer reported b that the first discovered protein kinase, phosphorylase kinase, ARCHITECTURE OF ABC KINASES was, itself, activated by phosphorylation [1]. Two decades later, Fischer and colleagues showed that the kinase that phosphory- Protein kinases A, B\Akt and C have in common a conserved lates phosphorylase kinase, later named protein kinase A (PKA), kinase core whose function is regulated allosterically by a was also phosphorylated [2]. Reversible control by phosphory- corresponding regulatory moiety (Figure 1). In the case of PKA, lation\dephosphorylation is now firmly established as a fun- the regulatory moiety is a separate polypeptide, whereas the damental mechanism for regulating the function of most of the regulatory determinants are on the same polypeptide as the 500 or so members of the mammalian protein kinase superfamily. -

Role of Ataxia-Telangiectasia Mutated Kinase in Cardiac Autophagy and Glucose Metabolism Under Ischemic Conditions Patsy Thrasher East Tennessee State University
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 8-2018 Role of Ataxia-Telangiectasia Mutated Kinase in Cardiac Autophagy and Glucose Metabolism Under Ischemic Conditions Patsy Thrasher East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Biology Commons, and the Physiology Commons Recommended Citation Thrasher, Patsy, "Role of Ataxia-Telangiectasia Mutated Kinase in Cardiac Autophagy and Glucose Metabolism Under Ischemic Conditions" (2018). Electronic Theses and Dissertations. Paper 3442. https://dc.etsu.edu/etd/3442 This Dissertation - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Role of Ataxia-Telangiectasia Mutated Kinase in Cardiac Autophagy and Glucose Metabolism Under Ischemic Conditions ____________________________________ A dissertation presented to the faculty of the Department of Biomedical Sciences East Tennessee State University In partial fulfillment of the requirements for the degree Doctor of Philosophy in Biomedical Sciences __________________________________ by Patsy R. Thrasher August 2018 _________________________________ Krishna Singh, Ph.D., Chair Mahipal Singh, Ph.D. Chuanfu Li, M.D. Tom Ecay, Ph.D. Yue Zou, Ph.D. Douglas Thewke, Ph.D. Keywords: ATM, myocardial infarction, autophagy, ischemia, glucose, metabolism ABSTRACT Role of Ataxia-Telangiectasia Mutated Kinase in Cardiac Autophagy and Glucose Metabolism Under Ischemic Conditions by Patsy R. Thrasher Ataxia-telangiectasia mutated kinase (ATM), a serine/threonine kinase primarily located in the nucleus, is typically activated in response to DNA damage. -

Paclitaxel Augments Cytotoxic Effect of Photodynamic Therapy Using Verteporfin in Gastric and Bile Duct Cancer Cells
PAPER www.rsc.org/pps | Photochemical & Photobiological Sciences Paclitaxel augments cytotoxic effect of photodynamic therapy using verteporfin in gastric and bile duct cancer cells Seungwoo Park,†a Sung Pil Hong,†a Tae Yoon Oh,b Seungmin Bang,a Jae Bock Chunga and Si Young Song*a,b Received 11th December 2007, Accepted 25th April 2008 First published as an Advance Article on the web 15th May 2008 DOI: 10.1039/b719072g Photodynamic therapy (PDT) shows a limited antitumor effect in treating gastrointestinal tumors because of improper light penetration or insufficient photosensitizer uptake. The aim of this study was to evaluate the cytotoxic effect of PDT combined with paclitaxel on in vitro cancer cells. In vitro photodynamic therapy was performed in gastric cancer cells (NCI-N87) and bile duct cancer cells (YGIC-6B) using verteporfin (2 ug mL−1) and a PTH light source (1 000 W, Oriel Co.) with 665–675 nm narrow band pass filter. Cytotoxicity was compared using the MTT assay between cancer cells treated with PDT alone or pretreated with paclitaxel (IC25). Apoptotic changes were evaluated using DAPI staining, DNA fragmentation analysis, Annexin V-FITC apoptosis assay, cell cycle analysis, and western blots for cytochrome c, Bax, and Bid. The PDT-induced cytotoxicity was potentiated by pretreating with low dose paclitaxel (P < 0.001). The enhanced cytotoxicity was due to an augmented apoptotic response mediated by exaggerated cytochrome c released from mitochondria, without Bax or Bid activation. These results show that paclitaxel pretreatment -

Photodynamic Therapy Induces Autophagy-Mediated Cell Death In
Song et al. Cell Death and Disease (2020) 11:938 https://doi.org/10.1038/s41419-020-03136-y Cell Death & Disease ARTICLE Open Access Photodynamic therapy induces autophagy- mediated cell death in human colorectal cancer cells via activation of the ROS/JNK signaling pathway Changfeng Song1,WenXu1,HongkunWu2,XiaotongWang1,QianyiGong1,ChangLiu2,JianwenLiu1 and Lin Zhou2 Abstract Evidence has shown that m-THPC and verteporfin (VP) are promising sensitizers in photodynamic therapy (PDT). In addition, autophagy can act as a tumor suppressor or a tumor promoter depending on the photosensitizer (PS) and the cancer cell type. However, the role of autophagy in m-THPC- and VP-mediated PDT in in vitro and in vivo models of human colorectal cancer (CRC) has not been reported. In this study, m-THPC-PDT or VP-PDT exhibited significant phototoxicity, inhibited proliferation, and induced the generation of large amounts of reactive oxygen species (ROS) in CRC cells. From immunoblotting, fluorescence image analysis, and transmission electron microscopy, we found extensive autophagic activation induced by ROS in cells. In addition, m-THPC-PDT or VP-PDT treatment significantly induced apoptosis in CRC cells. Interestingly, the inhibition of m-THPC-PDT-induced autophagy by knockdown of ATG5 or ATG7 substantially inhibited the apoptosis of CRC cells. Moreover, m-THPC- PDT treatment inhibited tumorigenesis of subcutaneous HCT116 xenografts. Meanwhile, antioxidant treatment 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; markedly inhibited autophagy and apoptosis induced by PDT in CRC cells by inactivating JNK signaling. In conclusion, inhibition of autophagy can remarkably alleviate PDT-mediated anticancer efficiency in CRC cells via inactivation of the ROS/JNK signaling pathway. -

And Glucocorticoid-Inducible Kinase (Sgk1)" (2007)
Dartmouth College Dartmouth Digital Commons Open Dartmouth: Published works by Dartmouth faculty Faculty Work 2-14-2007 Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid- Inducible Kinase (Sgk1) J. Denry Sato Mount Desert Island Biological Laboratory, Salisbury Cove M. Christine Chapline Dartmouth College Renee Thibodeau Dartmouth College Raymond A. Frizzell University of Pittsburgh Bruce A. Stanton Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Medicine and Health Sciences Commons Dartmouth Digital Commons Citation Sato, J. Denry; Chapline, M. Christine; Thibodeau, Renee; Frizzell, Raymond A.; and Stanton, Bruce A., "Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid-Inducible Kinase (Sgk1)" (2007). Open Dartmouth: Published works by Dartmouth faculty. 3156. https://digitalcommons.dartmouth.edu/facoa/3156 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Open Dartmouth: Published works by Dartmouth faculty by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. Original Paper Cellular Physiology Cell Physiol Biochem 2007;20:91-98 Accepted: February 14, 2007 and Biochemistry Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by Serum- and Glucocorticoid-Inducible Kinase (SGK1) J. Denry Sato1, M. Christine Chapline1,2, Renee Thibodeau1,2, Raymond A. Frizzell1,3 and Bruce A. Stanton1,2 1Mount Desert Island Biological Laboratory, Salisbury Cove, 2Dept. of Physiology, Dartmouth Medical School, Hanover, 3Dept. of Cell Biology and Physiology, University of Pittsburgh Medical School, Pittsburgh Key Words in stimulating CFTR Cl currents. -

Combination of Near Infrared Light-Activated Photodynamic Therapy Mediated by Indocyanine Green with Etoposide to Treat Non-Small-Cell Lung Cancer
cancers Article Combination of Near Infrared Light-Activated Photodynamic Therapy Mediated by Indocyanine Green with Etoposide to Treat Non-Small-Cell Lung Cancer Ting Luo 1, Qinrong Zhang 1,* and Qing-Bin Lu 1,2,* 1 Department of Physics and Astronomy, University of Waterloo, 200 University Avenue West, Waterloo, ON N2L 3G1, Canada; [email protected] 2 Departments of Biology and Chemistry, University of Waterloo, 200 University Avenue West, Waterloo, ON N2L 3G1, Canada * Correspondence: [email protected] (Q.Z.); [email protected] (Q.-B.L.); Tel.: +1-519-888-4567 (ext. 33503) (Q.-B.L.) Academic Editor: Michael R. Hamblin Received: 12 May 2017; Accepted: 1 June 2017; Published: 5 June 2017 Abstract: Indocyanine green (ICG) has been reported as a potential near-infrared (NIR) photosensitizer for photodynamic therapy (PDT) of cancer. However the application of ICG-mediated PDT is both intrinsically and physiologically limited. Here we report a combination of ICG-PDT with a chemotherapy drug etoposide (VP-16), aiming to enhance the anticancer efficacy, to circumvent limitations of PDT using ICG, and to reduce side effects of VP-16. We found in controlled in vitro cell-based assays that this combination is effective in killing non-small-cell lung cancer cells (NSCLC, A549 cell line). We also found that the combination of ICG-PDT and VP-16 exhibits strong synergy in killing non-small-cell lung cancer cells partially through inducing more DNA double-strand breaks (DSBs), while it has a much weaker synergy in killing human normal cells (GM05757). Furthermore, by studying the treatment sequence dependence and the cytotoxicity of laser-irradiated mixtures of ICG and VP-16, we found that the observed synergy involves direct/indirect reactions between ICG and VP-16. -

Targeting Phosphatidylinositol 3-Kinase Signaling Pathway For
Published OnlineFirst August 23, 2017; DOI: 10.1158/1535-7163.MCT-17-0326 Small Molecule Therapeutics Molecular Cancer Therapeutics Targeting Phosphatidylinositol 3-Kinase Signaling Pathway for Therapeutic Enhancement of Vascular-Targeted Photodynamic Therapy Daniel Kraus1, Pratheeba Palasuberniam1, and Bin Chen1,2 Abstract Vascular-targeted photodynamic therapy (PDT) selectively the strongest synergism, followed in order by combinations disrupts vascular function by inducing oxidative damages to with pan-PI3K inhibitor BKM120 and p110a isoform-selective the vasculature, particularly endothelial cells. Although effec- inhibitor BYL719. Combination treatments of PDT and tive tumor eradication and excellent safety profile are well BEZ235 exhibited a cooperative inhibition of antiapoptotic demonstrated in both preclinical and clinical studies, incom- Bcl-2 family protein Mcl-1 and induced more cell apoptosis plete vascular shutdown and angiogenesis are known to cause than each treatment alone. In addition to increasing treatment tumor recurrence after vascular-targeted PDT. We have explored lethality, BEZ235 combined with PDT effectively inhibited therapeutic enhancement of vascular-targeted PDT with PI3K PI3K pathway activation and consequent endothelial cell pro- signaling pathway inhibitors because the activation of PI3K liferation after PDT alone, leading to a sustained growth inhi- pathway was involved in promoting endothelial cell survival bition. In the PC-3 prostate tumor model, combination treat- and proliferation after PDT. Here, three clinically relevant ments improved treatment outcomes by turning a temporary small-molecule inhibitors (BYL719, BKM120, and BEZ235) of tumor regrowth delay induced by PDT alone to a more long- the PI3K pathway were evaluated in combination with verte- lasting treatment response. Our study strongly supports the porfin-PDT.