Protein Kinase C As a Paradigm Alexandra C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AGC Kinases in Mtor Signaling, in Mike Hall and Fuyuhiko Tamanoi: the Enzymes, Vol
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book, The Enzymes, Vol .27, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: ESTELA JACINTO, AGC Kinases in mTOR Signaling, In Mike Hall and Fuyuhiko Tamanoi: The Enzymes, Vol. 27, Burlington: Academic Press, 2010, pp.101-128. ISBN: 978-0-12-381539-2, © Copyright 2010 Elsevier Inc, Academic Press. Author's personal copy 7 AGC Kinases in mTOR Signaling ESTELA JACINTO Department of Physiology and Biophysics UMDNJ-Robert Wood Johnson Medical School, Piscataway New Jersey, USA I. Abstract The mammalian target of rapamycin (mTOR), a protein kinase with homology to lipid kinases, orchestrates cellular responses to growth and stress signals. Various extracellular and intracellular inputs to mTOR are known. mTOR processes these inputs as part of two mTOR protein com- plexes, mTORC1 or mTORC2. Surprisingly, despite the many cellular functions that are linked to mTOR, there are very few direct mTOR substrates identified to date. -
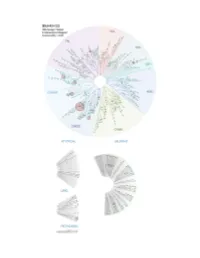
Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) BSJ-03-123 AAK1 AAK1 94 1000 BSJ-03-123 ABL1(E255K)-phosphorylated ABL1 79 1000 BSJ-03-123 ABL1(F317I)-nonphosphorylated ABL1 89 1000 BSJ-03-123 ABL1(F317I)-phosphorylated ABL1 98 1000 BSJ-03-123 ABL1(F317L)-nonphosphorylated ABL1 86 1000 BSJ-03-123 ABL1(F317L)-phosphorylated ABL1 89 1000 BSJ-03-123 ABL1(H396P)-nonphosphorylated ABL1 76 1000 BSJ-03-123 ABL1(H396P)-phosphorylated ABL1 90 1000 BSJ-03-123 ABL1(M351T)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1(Q252H)-nonphosphorylated ABL1 56 1000 BSJ-03-123 ABL1(Q252H)-phosphorylated ABL1 97 1000 BSJ-03-123 ABL1(T315I)-nonphosphorylated ABL1 100 1000 BSJ-03-123 ABL1(T315I)-phosphorylated ABL1 85 1000 BSJ-03-123 ABL1(Y253F)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1-nonphosphorylated ABL1 60 1000 BSJ-03-123 ABL1-phosphorylated ABL1 79 1000 BSJ-03-123 ABL2 ABL2 89 1000 BSJ-03-123 ACVR1 ACVR1 100 1000 BSJ-03-123 ACVR1B ACVR1B 95 1000 BSJ-03-123 ACVR2A ACVR2A 100 1000 BSJ-03-123 ACVR2B ACVR2B 96 1000 BSJ-03-123 ACVRL1 ACVRL1 84 1000 BSJ-03-123 ADCK3 CABC1 90 1000 BSJ-03-123 ADCK4 ADCK4 91 1000 BSJ-03-123 AKT1 AKT1 100 1000 BSJ-03-123 AKT2 AKT2 98 1000 BSJ-03-123 AKT3 AKT3 100 1000 BSJ-03-123 ALK ALK 100 1000 BSJ-03-123 ALK(C1156Y) ALK 78 1000 BSJ-03-123 ALK(L1196M) ALK 100 1000 BSJ-03-123 AMPK-alpha1 PRKAA1 93 1000 BSJ-03-123 AMPK-alpha2 PRKAA2 100 1000 BSJ-03-123 ANKK1 ANKK1 89 1000 BSJ-03-123 ARK5 NUAK1 98 1000 BSJ-03-123 ASK1 MAP3K5 100 1000 BSJ-03-123 ASK2 MAP3K6 92 1000 BSJ-03-123 AURKA -

Glycogen Synthase Kinase-3 Is Activated in Neuronal Cells by G 12
The Journal of Neuroscience, August 15, 2002, 22(16):6863–6875 Glycogen Synthase Kinase-3 Is Activated in Neuronal Cells by G␣ ␣ 12 and G 13 by Rho-Independent and Rho-Dependent Mechanisms C. Laura Sayas, Jesu´ s Avila, and Francisco Wandosell Centro de Biologı´a Molecular “Severo Ochoa”, Consejo Superior de Investigaciones Cientı´ficas, Universidad Auto´ noma de Madrid, Cantoblanco, Madrid 28049, Spain ␣ ␣ ␣ ␣ Glycogen synthase kinase-3 (GSK-3) was generally considered tively active G 12 (G 12QL) and G 13 (G 13QL) in Neuro2a cells a constitutively active enzyme, only regulated by inhibition. induces upregulation of GSK-3 activity. Furthermore, overex- Here we describe that GSK-3 is activated by lysophosphatidic pression of constitutively active RhoA (RhoAV14) also activates ␣ acid (LPA) during neurite retraction in rat cerebellar granule GSK-3 However, the activation of GSK-3 by G 13 is blocked by neurons. GSK-3 activation correlates with an increase in GSK-3 coexpression with C3 transferase, whereas C3 does not block ␣ tyrosine phosphorylation. In addition, LPA induces a GSK-3- GSK-3 activation by G 12. Thus, we demonstrate that GSK-3 is ␣ ␣ mediated hyperphosphorylation of the microtubule-associated activated by both G 12 and G 13 in neuronal cells. However, ␣ protein tau. Inhibition of GSK-3 by lithium partially blocks neu- GSK-3 activation by G 13 is Rho-mediated, whereas GSK-3 ␣ rite retraction, indicating that GSK-3 activation is important but activation by G 12 is Rho-independent. The results presented not essential for the neurite retraction progress. GSK-3 activa- here imply the existence of a previously unknown mechanism of ␣ tion by LPA in cerebellar granule neurons is neither downstream GSK-3 activation by G 12/13 subunits. -

PRKCQ Promotes Oncogenic Growth and Anoikis Resistance of a Subset
Byerly et al. Breast Cancer Research (2016) 18:95 DOI 10.1186/s13058-016-0749-6 RESEARCH ARTICLE Open Access PRKCQ promotes oncogenic growth and anoikis resistance of a subset of triple- negative breast cancer cells Jessica Byerly1†, Gwyneth Halstead-Nussloch1†, Koichi Ito1, Igor Katsyv3 and Hanna Y. Irie1,2* Abstract Background: The protein kinase C (PKC) family comprises distinct classes of proteins, many of which are implicated in diverse cellular functions. Protein tyrosine kinase C theta isoform (PRKCQ)/PKCθ, a member of the novel PKC family, may have a distinct isoform-specific role in breast cancer. PKCθ is preferentially expressed in triple-negative breast cancer (TNBC) compared to other breast tumor subtypes. We hypothesized that PRKCQ/PKCθ critically regulates growth and survival of a subset of TNBC cells. Methods: To elucidate the role of PRKCQ/PKCθ in regulating growth and anoikis resistance, we used both gain and loss of function to modulate expression of PRKCQ. We enhanced expression of PKCθ (kinase-active or inactive) in non-transformed breast epithelial cells (MCF-10A) and assessed effects on epidermal growth factor (EGF)- independent growth, anoikis, and migration. We downregulated expression of PKCθ in TNBC cells, and determined effects on in vitro and in vivo growth and survival. TNBC cells were also treated with a small molecule inhibitor to assess requirement for PKCθ kinase activity in the growth of TNBC cells. Results: PRKCQ/PKCθ can promote oncogenic phenotypes when expressed in non-transformed MCF-10A mammary epithelial cells; PRKCQ/PKCθ enhances anchorage-independent survival, growth-factor-independent proliferation, and migration. PKCθ expression promotes retinoblastoma (Rb) phosphorylation and cell-cycle progression under growth factor-deprived conditions that typically induce cell-cycle arrest of MCF-10A breast epithelial cells. -

Table S1. List of Oligonucleotide Primers Used
Table S1. List of oligonucleotide primers used. Cla4 LF-5' GTAGGATCCGCTCTGTCAAGCCTCCGACC M629Arev CCTCCCTCCATGTACTCcgcGATGACCCAgAGCTCGTTG M629Afwd CAACGAGCTcTGGGTCATCgcgGAGTACATGGAGGGAGG LF-3' GTAGGCCATCTAGGCCGCAATCTCGTCAAGTAAAGTCG RF-5' GTAGGCCTGAGTGGCCCGAGATTGCAACGTGTAACC RF-3' GTAGGATCCCGTACGCTGCGATCGCTTGC Ukc1 LF-5' GCAATATTATGTCTACTTTGAGCG M398Arev CCGCCGGGCAAgAAtTCcgcGAGAAGGTACAGATACGc M398Afwd gCGTATCTGTACCTTCTCgcgGAaTTcTTGCCCGGCGG LF-3' GAGGCCATCTAGGCCATTTACGATGGCAGACAAAGG RF-5' GTGGCCTGAGTGGCCATTGGTTTGGGCGAATGGC RF-3' GCAATATTCGTACGTCAACAGCGCG Nrc2 LF-5' GCAATATTTCGAAAAGGGTCGTTCC M454Grev GCCACCCATGCAGTAcTCgccGCAGAGGTAGAGGTAATC M454Gfwd GATTACCTCTACCTCTGCggcGAgTACTGCATGGGTGGC LF-3' GAGGCCATCTAGGCCGACGAGTGAAGCTTTCGAGCG RF-5' GAGGCCTGAGTGGCCTAAGCATCTTGGCTTCTGC RF-3' GCAATATTCGGTCAACGCTTTTCAGATACC Ipl1 LF-5' GTCAATATTCTACTTTGTGAAGACGCTGC M629Arev GCTCCCCACGACCAGCgAATTCGATagcGAGGAAGACTCGGCCCTCATC M629Afwd GATGAGGGCCGAGTCTTCCTCgctATCGAATTcGCTGGTCGTGGGGAGC LF-3' TGAGGCCATCTAGGCCGGTGCCTTAGATTCCGTATAGC RF-5' CATGGCCTGAGTGGCCGATTCTTCTTCTGTCATCGAC RF-3' GACAATATTGCTGACCTTGTCTACTTGG Ire1 LF-5' GCAATATTAAAGCACAACTCAACGC D1014Arev CCGTAGCCAAGCACCTCGgCCGAtATcGTGAGCGAAG D1014Afwd CTTCGCTCACgATaTCGGcCGAGGTGCTTGGCTACGG LF-3' GAGGCCATCTAGGCCAACTGGGCAAAGGAGATGGA RF-5' GAGGCCTGAGTGGCCGTGCGCCTGTGTATCTCTTTG RF-3' GCAATATTGGCCATCTGAGGGCTGAC Kin28 LF-5' GACAATATTCATCTTTCACCCTTCCAAAG L94Arev TGATGAGTGCTTCTAGATTGGTGTCggcGAAcTCgAGCACCAGGTTG L94Afwd CAACCTGGTGCTcGAgTTCgccGACACCAATCTAGAAGCACTCATCA LF-3' TGAGGCCATCTAGGCCCACAGAGATCCGCTTTAATGC RF-5' CATGGCCTGAGTGGCCAGGGCTAGTACGACCTCG -

Protein Kinases Phosphorylation/Dephosphorylation Protein Phosphorylation Is One of the Most Important Mechanisms of Cellular Re
Protein Kinases Phosphorylation/dephosphorylation Protein phosphorylation is one of the most important mechanisms of cellular responses to growth, stress metabolic and hormonal environmental changes. Most mammalian protein kinases have highly a homologous 30 to 32 kDa catalytic domain. • Most common method of reversible modification - activation and localization • Up to 1/3 of cellular proteins can be phosphorylated • Leads to a very fast response to cellular stress, hormonal changes, learning processes, transcription regulation .... • Different than allosteric or Michealis Menten regulation Protein Kinome To date – 518 human kinases known • 50 kinase families between yeast, invertebrate and mammaliane kinomes • 518 human PKs, most (478) belong to single super family whose catalytic domain are homologous. • Kinase dendrogram displays relative similarities based on catalytic domains. • AGC (PKA, PKG, PKC) • CAMK (Casein kinase 1) • CMGC (CDC, MAPK, GSK3, CLK) • STE (Sterile 7, 11 & 20 kinases) • TK (Tryosine kinases memb and cyto) • TKL (Tyrosine kinase-like) • Phosphorylation stabilized thermodynamically - only half available energy used in adding phosphoryl to protein - change in free energy forces phosphorylation reaction in one direction • Phosphatases reverse direction • The rate of reaction of most phosphatases are 1000 times faster • Phosphorylation occurs on Ser/The or Tyr • What differences occur due to the addition of a phosphoryl group? • Regulation of protein phosphorylation varies depending on protein - some turned on or off -

Distinct Regulation of Cardiac Fibroblast Proliferation and Transdifferentiation by Classical and Novel Protein Kinase C Isoform
Molecular Pharmacology Fast Forward. Published on November 25, 2020 as DOI: 10.1124/molpharm.120.000094 This article has not been copyedited and formatted. The final version may differ from this version. Distinct regulation of cardiac fibroblast proliferation and transdifferentiation by classical and novel protein kinase C isoforms: possible implications for new antifibrotic therapies S. Tuuli Karhu, Heikki Ruskoaho, Virpi Talman* Affiliations Downloaded from STK, HR, and VT: Drug Research Program and Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki, Finland molpharm.aspetjournals.org *Corresponding author at ASPET Journals on September 30, 2021 1 Molecular Pharmacology Fast Forward. Published on November 25, 2020 as DOI: 10.1124/molpharm.120.000094 This article has not been copyedited and formatted. The final version may differ from this version. Running title: PKC agonists inhibit cardiac fibroblast activation Corresponding author: Virpi Talman Division of Pharmacology and Pharmacotherapy Faculty of Pharmacy University of Helsinki P.O. Box 56 (Viikinkaari 5E) FI-00014 Helsinki, FINLAND Downloaded from Tel: +358504480768 Email: [email protected] molpharm.aspetjournals.org Number of text pages: 35 Number of tables: 0 Number of figures: 4 at ASPET Journals on September 30, 2021 Number of references: 55 Number of words in the Abstract: 249 Number of words in the Introduction: 728 Number of words in the Discussion: 1459 Abbreviations: α-SMA, α-smooth muscle actin; aPKC, atypical protein kinase C isoform; BrdU, 5-bromo-2’-deoxyuridine; CF, cardiac fibroblast; cPKC, classical protein kinase C isoform; DDR2, discoidin domain receptor 2; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; HCA, high-content analysis; LDH, lactate dehydrogenase; MTT, 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; nPKC, novel protein kinase C isoform; PKC, protein kinase C 2 Molecular Pharmacology Fast Forward. -

And Glucocorticoid-Inducible Kinase (Sgk1)" (2007)
Dartmouth College Dartmouth Digital Commons Open Dartmouth: Published works by Dartmouth faculty Faculty Work 2-14-2007 Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid- Inducible Kinase (Sgk1) J. Denry Sato Mount Desert Island Biological Laboratory, Salisbury Cove M. Christine Chapline Dartmouth College Renee Thibodeau Dartmouth College Raymond A. Frizzell University of Pittsburgh Bruce A. Stanton Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Medicine and Health Sciences Commons Dartmouth Digital Commons Citation Sato, J. Denry; Chapline, M. Christine; Thibodeau, Renee; Frizzell, Raymond A.; and Stanton, Bruce A., "Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (Cftr) by Serum- and Glucocorticoid-Inducible Kinase (Sgk1)" (2007). Open Dartmouth: Published works by Dartmouth faculty. 3156. https://digitalcommons.dartmouth.edu/facoa/3156 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Open Dartmouth: Published works by Dartmouth faculty by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. Original Paper Cellular Physiology Cell Physiol Biochem 2007;20:91-98 Accepted: February 14, 2007 and Biochemistry Regulation of Human Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by Serum- and Glucocorticoid-Inducible Kinase (SGK1) J. Denry Sato1, M. Christine Chapline1,2, Renee Thibodeau1,2, Raymond A. Frizzell1,3 and Bruce A. Stanton1,2 1Mount Desert Island Biological Laboratory, Salisbury Cove, 2Dept. of Physiology, Dartmouth Medical School, Hanover, 3Dept. of Cell Biology and Physiology, University of Pittsburgh Medical School, Pittsburgh Key Words in stimulating CFTR Cl currents. -

Protein Kinase C As a Therapeutic Target in Non-Small Cell Lung Cancer
International Journal of Molecular Sciences Review Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer Mohammad Mojtaba Sadeghi 1,2, Mohamed F. Salama 2,3,4 and Yusuf A. Hannun 1,2,3,* 1 Department of Biochemistry, Molecular and Cellular Biology, Stony Brook University, Stony Brook, NY 11794, USA; [email protected] 2 Stony Brook Cancer Center, Stony Brook University Hospital, Stony Brook, NY 11794, USA; [email protected] 3 Department of Medicine, Stony Brook University, Stony Brook, NY 11794, USA 4 Department of Biochemistry, Faculty of Veterinary Medicine, Mansoura University, Mansoura 35516, Dakahlia Governorate, Egypt * Correspondence: [email protected] Abstract: Driver-directed therapeutics have revolutionized cancer treatment, presenting similar or better efficacy compared to traditional chemotherapy and substantially improving quality of life. Despite significant advances, targeted therapy is greatly limited by resistance acquisition, which emerges in nearly all patients receiving treatment. As a result, identifying the molecular modulators of resistance is of great interest. Recent work has implicated protein kinase C (PKC) isozymes as mediators of drug resistance in non-small cell lung cancer (NSCLC). Importantly, previous findings on PKC have implicated this family of enzymes in both tumor-promotive and tumor-suppressive biology in various tissues. Here, we review the biological role of PKC isozymes in NSCLC through extensive analysis of cell-line-based studies to better understand the rationale for PKC inhibition. Citation: Sadeghi, M.M.; Salama, PKC isoforms α, ", η, ι, ζ upregulation has been reported in lung cancer, and overexpression correlates M.F.; Hannun, Y.A. Protein Kinase C with worse prognosis in NSCLC patients. -

AMP-Activated Protein Kinase: the Current Landscape for Drug Development
REVIEWS AMP-activated protein kinase: the current landscape for drug development Gregory R. Steinberg 1* and David Carling2 Abstract | Since the discovery of AMP-activated protein kinase (AMPK) as a central regulator of energy homeostasis, many exciting insights into its structure, regulation and physiological roles have been revealed. While exercise, caloric restriction, metformin and many natural products increase AMPK activity and exert a multitude of health benefits, developing direct activators of AMPK to elicit beneficial effects has been challenging. However, in recent years, direct AMPK activators have been identified and tested in preclinical models, and a small number have entered clinical trials. Despite these advances, which disease(s) represent the best indications for therapeutic AMPK activation and the long-term safety of such approaches remain to be established. Cardiovascular disease Dramatic improvements in health care coupled with identifying a unifying mechanism that could link these (CVD). A term encompassing an increased standard of living, including better nutri- changes to multiple branches of metabolism followed diseases affecting the heart tion and education, have led to a remarkable increase in the discovery that the AMP-activated protein kinase or circulatory system. human lifespan1. Importantly, the number of years spent (AMPK) provided a common regulatory mechanism in good health is also increasing2. Despite these positive for inhibiting both cholesterol (through phosphoryla- Non-alcoholic fatty liver disease developments, there are substantial risks that challenge tion of HMG-CoA reductase (HMGR)) and fatty acid (NAFLD). A very common continued improvements in human health. Perhaps the (through phosphorylation of acetyl-CoA carboxylase disease in humans in which greatest threat to future health is a chronic energy imbal- (ACC)) synthesis8 (BOx 1). -

A Cell-Based Screening to Detect Inhibitors of BRAF Signaling Pathway
The Journal of Antibiotics (2009) 62, 105–107 & 2009 Japan Antibiotics Research Association All rights reserved 0021-8820/09 $32.00 www.nature.com/ja NOTE A cell-based screening to detect inhibitors of BRAF signaling pathway Yukihiro Asami1,3, Mihoko Mori1,4, Hiroyuki Koshino2, Yasuyo Sekiyama1,5, Takayuki Teruya1, Siro Simizu1, Takeo Usui1,6 and Hiroyuki Osada1 The Journal of Antibiotics (2009) 62, 105–107; doi:10.1038/ja.2008.17; published online 9 January 2009 Keywords: BRAF; cell-based assay; malformins; protein phosphatase; signal transduction BRAF, a serine/threonine kinase, regulates the mitogen-activated inhibitor)—on the cell-based assay.4,5 We found that U0126 and protein kinase (RAS/RAF/MEK/ERK) pathway, which is involved in geldanamycin preferentially decreased the cell viability of BRAF the transduction of mitogenic signals from the cell membrane to the mutant WM266-4 cells (Table 1). The selectivity ratio of CHL-1/ nucleus. BRAF is known to be mutated in 70% of malignant WM266-4 was more than 10-fold. The selectivity ratios of other melanomas. The most common BRAF mutation is V600E, and it inhibitors were smaller than 1.0. These results suggest that this increases the kinase activity that constitutively stimulates ERK screening system is suitable for the detection of BRAF-signaling signaling, cell proliferation and survival. The mutation is an early inhibitors from microbial metabolites. event for the development of melanoma and it is present in 80% of In this cell-based assay, we tested 3000 microbial extracts and primary melanomas.1,2 Recently, it has been reported that mutant identified the aimed inhibitory activity in the fermentation broth of BRAF protein is a client of Hsp90, which is required for the folding an unidentified fungus. -

Protein Kinase C Is a Key Target for Attenuation of Leigh Syndrome by Rapamycin Authors: Miguel Martin-Perez1†#, Takashi K
bioRxiv preprint doi: https://doi.org/10.1101/562207; this version posted February 27, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Title: Protein kinase C is a key target for attenuation of Leigh syndrome by rapamycin Authors: Miguel Martin-Perez1†#, Takashi K. Ito2‡#, Anthony S. Grillo2, Anthony S. Valente1, Jeehae Han2, Samuel Entwisle1,3, Heather Z. Huang2, Dayae Kim2, Masanao Yajima4, Matt 5 Kaeberlein2,3,*, Judit Villén1,3,*. Affiliations: 1Department of Genome Sciences, University of Washington, Seattle, United States. 2Department of Pathology, University of Washington, Seattle, United States. 3Molecular and Cellular Biology, University of Washington, Seattle, United States. 10 4Department of Mathematics and Statistics, Boston University, Boston, MA, United States. *Correspondence to: [email protected], [email protected] †Current address: Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology (BIST), Barcelona, Spain. ‡Current address: International Mass Imaging Center, Hamamatsu University School of 15 Medicine, Hamamatsu, Japan. #These authors contributed equally to this work. Abstract: Leigh syndrome is a fatal neurometabolic disorder caused by defects in mitochondrial function. mTOR inhibition with rapamycin attenuates disease progression in a mouse model of Leigh syndrome (Ndufs4 KO mouse); however, the mechanism of rescue is unknown. Here we 20 assessed the impact of rapamycin on the brain proteome and phosphoproteome of Ndufs4 KO mice. We report that rapamycin remodels the brain proteome to alter mitochondrial structure, inhibits signaling through both mTOR complexes, and inhibits multiple protein kinase C (PKC) isoforms.