Organic Cofactors Participated More Frequently Than Transition Metals in Redox Reactions of Primitive Proteins Hong-Fang Ji, Lei Chen, and Hong-Yu Zhang*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amplitaq and Amplitaq Gold DNA Polymerase
AmpliTaq and AmpliTaq Gold DNA Polymerase The Most Referenced Brand of DNA Polymerase in the World Date: 2005-05 Notes: Authors are listed alphabetically J. Biol. Chem. (223) Ezoe, S., I. Matsumura, et al. (2005). "GATA Transcription Factors Inhibit Cytokine-dependent Growth and Survival of a Hematopoietic Cell Line through the Inhibition of STAT3 Activity." J. Biol. Chem. 280(13): 13163-13170. http://www.jbc.org/cgi/content/abstract/280/13/13163 Although GATA-1 and GATA-2 were shown to be essential for the development of hematopoietic cells by gene targeting experiments, they were also reported to inhibit the growth of hematopoietic cells. Therefore, in this study, we examined the effects of GATA-1 and GATA-2 on cytokine signals. A tamoxifen-inducible form of GATA-1 (GATA-1/ERT) showed a minor inhibitory effect on interleukin-3 (IL-3)-dependent growth of an IL-3-dependent cell line Ba/F3. On the other hand, it drastically inhibited TPO-dependent growth and gp130-mediated growth/survival of Ba/F3. Similarly, an estradiol-inducible form of GATA-2 (GATA-2/ER) disrupted thrombopoietin (TPO)-dependent growth and gp130-mediated growth/survival of Ba/F3. As for this mechanism, we found that both GATA-1 and GATA-2 directly bound to STAT3 both in vitro and in vivo and inhibited its DNA-binding activity in gel shift assays and chromatin immunoprecipitation assays, whereas they hardly affected STAT5 activity. In addition, endogenous GATA-1 was found to interact with STAT3 in normal megakaryocytes, suggesting that GATA-1 may inhibit STAT3 activity in normal hematopoietic cells. -

CYP2A6) by P53
Transcriptional Regulation of Human Stress Responsive Cytochrome P450 2A6 (CYP2A6) by p53 Hao Hu M.Biotech. (Biotechnology) 2012 The University of Queensland B.B.A. 2009 University of Electronic Science and Technology of China B.Sc. (Pharmacy) 2009 University of Electronic Science and Technology of China A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Medicine ABSTRACT Human cytochrome P450 (CYP) 2A6 is highly expressed in the liver and the encoding gene is regulated by various stress activated transcription factors, such as the nuclear factor (erythroid-derived 2)-like 2 (Nrf-2). Unlike the other xenobiotic metabolising CYP enzymes (XMEs), CYP2A6 only plays a minor role in xenobiotic metabolism. The CYP2A6 is highly induced by multiple forms of cellular stress conditions, where XMEs expression is normally inhibited. Recent findings suggest that the CYP2A6 plays an important role in regulating BR homeostasis. A computer based sequence analysis on the 3 kb proximate CYP2A6 promoter revealed several putative binding sites for p53, a protein that mediates regulation of antioxidant and apoptosis pathways. In this study, the role of p53 in CYP2A6 gene regulation is demonstrated. The site closest to transcription start site (TSS) is highly homologous with the p53 consensus sequence. The p53 responsiveness of this site was confirmed by transfections with various stepwise deleted of CYP2A6-5’-Luc constructs containing the putative p53RE. Deletion of the putative p53RE resulted in a total abolishment of p53 responsiveness of CYP2A6 promoter. Specific binding of p53 to the putative p53RE was detected by electrophoresis mobility shift assay. -

Characterisation of Bilirubin Metabolic Pathway in Hepatic Mitochondria Siti Nur Fadzilah Muhsain M.Sc
Characterisation of Bilirubin Metabolic Pathway in Hepatic Mitochondria Siti Nur Fadzilah Muhsain M.Sc. (Medical Research) 2005 Universiti Sains Malaysia Postgrad. Dip. (Toxicology) 2003 University of Surrey B.Sc.(Biomed. Sc.) 2000 Universiti Putra Malaysia A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2014 School of Medicine ABSTRACT Bilirubin (BR), a toxic waste product of degraded haem, is a potent antioxidant at physiological concentrations. To achieve the maximum benefit of BR, its intracellular level needs to be carefully regulated. A system comprising of two enzymes, haem oxygenase-1 (HMOX1) and cytochrome P450 2A5 (CYP2A5) exists in the endoplasmic reticulum (ER), responsible for regulating BR homeostasis. This system is induced in response to oxidative stress. In this thesis, oxidative stress caused accumulation of these enzymes in mitochondria — major producers and targets of reactive oxygen species (ROS) — is demonstrated. To understand the significance of this intracellular targeting, properties of microsomal and mitochondrial BR metabolising enzymes were compared and the capacity of mitochondrial CYP2A5 to oxidise BR in response to oxidative stress is reported. Microsomes and mitochondrial fractions were isolated from liver homogenates of DBA/2J mice, administered with sub-toxic dose of pyrazole, an oxidant stressor. The purity of extracted organelles was determined by analysing the expressions and activities of their respective marker enzymes. HMOX1 and CYP2A5 were significantly increased in microsomes and even more so in mitochondria in response to pyrazole-induced oxidative stress. By contrast, the treatment did not increase either microsomes or mitochondrial Uridine-diphosphate-glucuronosyltransferase 1A1 (UGT1A1), the sole enzyme that catalyses BR elimination through glucuronidation. -

Heme Oxygenase-1 Regulates Mitochondrial Quality Control in the Heart
RESEARCH ARTICLE Heme oxygenase-1 regulates mitochondrial quality control in the heart Travis D. Hull,1,2 Ravindra Boddu,1,3 Lingling Guo,2,3 Cornelia C. Tisher,1,3 Amie M. Traylor,1,3 Bindiya Patel,1,4 Reny Joseph,1,3 Sumanth D. Prabhu,1,4,5 Hagir B. Suliman,6 Claude A. Piantadosi,7 Anupam Agarwal,1,3,5 and James F. George2,3,4 1Department of Medicine, 2Department of Surgery, 3Nephrology Research and Training Center, and 4Comprehensive Cardiovascular Center, University of Alabama at Birmingham, Birmingham, Alabama, USA. 5Department of Veterans Affairs, Birmingham, Alabama, USA. 6Department of Anesthesiology and 7Department of Pulmonary, Allergy and Critical Care, Duke University School of Medicine, Durham, North Carolina, USA. The cardioprotective inducible enzyme heme oxygenase-1 (HO-1) degrades prooxidant heme into equimolar quantities of carbon monoxide, biliverdin, and iron. We hypothesized that HO-1 mediates cardiac protection, at least in part, by regulating mitochondrial quality control. We treated WT and HO-1 transgenic mice with the known mitochondrial toxin, doxorubicin (DOX). Relative to WT mice, mice globally overexpressing human HO-1 were protected from DOX-induced dilated cardiomyopathy, cardiac cytoarchitectural derangement, and infiltration of CD11b+ mononuclear phagocytes. Cardiac-specific overexpression of HO-1 ameliorated DOX-mediated dilation of the sarcoplasmic reticulum as well as mitochondrial disorganization in the form of mitochondrial fragmentation and increased numbers of damaged mitochondria in autophagic vacuoles. HO-1 overexpression promotes mitochondrial biogenesis by upregulating protein expression of NRF1, PGC1α, and TFAM, which was inhibited in WT animals treated with DOX. Concomitantly, HO-1 overexpression inhibited the upregulation of the mitochondrial fission mediator Fis1 and resulted in increased expression of the fusion mediators, Mfn1 and Mfn2. -

CDH12 Cadherin 12, Type 2 N-Cadherin 2 RPL5 Ribosomal
5 6 6 5 . 4 2 1 1 1 2 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 A A A A A A A A A A A A A A A A A A A A C C C C C C C C C C C C C C C C C C C C R R R R R R R R R R R R R R R R R R R R B , B B B B B B B B B B B B B B B B B B B , 9 , , , , 4 , , 3 0 , , , , , , , , 6 2 , , 5 , 0 8 6 4 , 7 5 7 0 2 8 9 1 3 3 3 1 1 7 5 0 4 1 4 0 7 1 0 2 0 6 7 8 0 2 5 7 8 0 3 8 5 4 9 0 1 0 8 8 3 5 6 7 4 7 9 5 2 1 1 8 2 2 1 7 9 6 2 1 7 1 1 0 4 5 3 5 8 9 1 0 0 4 2 5 0 8 1 4 1 6 9 0 0 6 3 6 9 1 0 9 0 3 8 1 3 5 6 3 6 0 4 2 6 1 0 1 2 1 9 9 7 9 5 7 1 5 8 9 8 8 2 1 9 9 1 1 1 9 6 9 8 9 7 8 4 5 8 8 6 4 8 1 1 2 8 6 2 7 9 8 3 5 4 3 2 1 7 9 5 3 1 3 2 1 2 9 5 1 1 1 1 1 1 5 9 5 3 2 6 3 4 1 3 1 1 4 1 4 1 7 1 3 4 3 2 7 6 4 2 7 2 1 2 1 5 1 6 3 5 6 1 3 6 4 7 1 6 5 1 1 4 1 6 1 7 6 4 7 e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m -
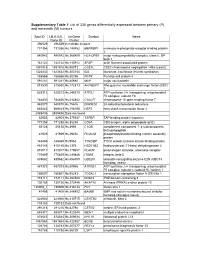
Supplementary Table 1 List of 335 Genes Differentially Expressed Between Primary (P) and Metastatic (M) Tumours
Supplementary Table 1 List of 335 genes differentially expressed between primary (P) and metastatic (M) tumours Spot ID I.M.A.G.E. UniGene Symbol Name Clone ID Cluster 296529 296529 In multiple clusters 731356 731356 Hs.140452 M6PRBP1 mannose-6-phosphate receptor binding protein 1 840942 840942 Hs.368409 HLA-DPB1 major histocompatibility complex, class II, DP beta 1 142122 142122 Hs.115912 AFAP actin filament associated protein 1891918 1891918 Hs.90073 CSE1L CSE1 chromosome segregation 1-like (yeast) 1323432 1323432 Hs.303154 IDS iduronate 2-sulfatase (Hunter syndrome) 788566 788566 Hs.80296 PCP4 Purkinje cell protein 4 591281 591281 Hs.80680 MVP major vault protein 815530 815530 Hs.172813 ARHGEF7 Rho guanine nucleotide exchange factor (GEF) 7 825312 825312 Hs.246310 ATP5J ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F6 784830 784830 Hs.412842 C10orf7 chromosome 10 open reading frame 7 840878 840878 Hs.75616 DHCR24 24-dehydrocholesterol reductase 669443 669443 Hs.158195 HSF2 heat shock transcription factor 2 2485436 2485436 Data not found 82903 82903 Hs.370937 TAPBP TAP binding protein (tapasin) 771258 771258 Hs.85258 CD8A CD8 antigen, alpha polypeptide (p32) 85128 85128 Hs.8986 C1QB complement component 1, q subcomponent, beta polypeptide 41929 41929 Hs.39252 PICALM phosphatidylinositol binding clathrin assembly protein 148469 148469 Hs.9963 TYROBP TYRO protein tyrosine kinase binding protein 415145 415145 Hs.1376 HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 810017 810017 Hs.179657 PLAUR plasminogen activator, -

Biocatalyzed Synthesis of Statins: a Sustainable Strategy for the Preparation of Valuable Drugs
catalysts Review Biocatalyzed Synthesis of Statins: A Sustainable Strategy for the Preparation of Valuable Drugs Pilar Hoyos 1, Vittorio Pace 2 and Andrés R. Alcántara 1,* 1 Department of Chemistry in Pharmaceutical Sciences, Faculty of Pharmacy, Complutense University of Madrid, Campus de Moncloa, E-28040 Madrid, Spain; [email protected] 2 Department of Pharmaceutical Chemistry, Faculty of Life Sciences, Althanstrasse 14, A-1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +34-91-394-1823 Received: 25 February 2019; Accepted: 9 March 2019; Published: 14 March 2019 Abstract: Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are the largest selling class of drugs prescribed for the pharmacological treatment of hypercholesterolemia and dyslipidaemia. Statins also possess other therapeutic effects, called pleiotropic, because the blockade of the conversion of HMG-CoA to (R)-mevalonate produces a concomitant inhibition of the biosynthesis of numerous isoprenoid metabolites (e.g., geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP)). Thus, the prenylation of several cell signalling proteins (small GTPase family members: Ras, Rac, and Rho) is hampered, so that these molecular switches, controlling multiple pathways and cell functions (maintenance of cell shape, motility, factor secretion, differentiation, and proliferation) are regulated, leading to beneficial effects in cardiovascular health, regulation of the immune system, anti-inflammatory and immunosuppressive properties, prevention and treatment of sepsis, treatment of autoimmune diseases, osteoporosis, kidney and neurological disorders, or even in cancer therapy. Thus, there is a growing interest in developing more sustainable protocols for preparation of statins, and the introduction of biocatalyzed steps into the synthetic pathways is highly advantageous—synthetic routes are conducted under mild reaction conditions, at ambient temperature, and can use water as a reaction medium in many cases. -

Identification of Systemic Immune Response Markers Through
Gray et al. Veterinary Research (2015) 46:7 DOI 10.1186/s13567-014-0138-z VETERINARY RESEARCH RESEARCH Open Access Identification of systemic immune response markers through metabolomic profiling of plasma from calves given an intra-nasally delivered respiratory vaccine Darren W Gray1*, Michael D Welsh2, Simon Doherty2, Fawad Mansoor2, Olivier P Chevallier1, Christopher T Elliott1 and Mark H Mooney1 Abstract Vaccination procedures within the cattle industry are important disease control tools to minimize economic and welfare burdens associated with respiratory pathogens. However, new vaccine, antigen and carrier technologies are required to combat emerging viral strains and enhance the efficacy of respiratory vaccines, particularly at the point of pathogen entry. New technologies, specifically metabolomic profiling, could be applied to identify metabolite immune-correlates representative of immune protection following vaccination aiding in the design and screening of vaccine candidates. This study for the first time demonstrates the ability of untargeted UPLC-MS metabolomic profiling to identify metabolite immune correlates characteristic of immune responses following mucosal vaccination in calves. Male Holstein Friesian calves were vaccinated with Pfizer Rispoval® PI3 + RSV intranasal vaccine and metabolomic profiling of post-vaccination plasma revealed 12 metabolites whose peak intensities differed significantly from controls. Plasma levels of glycocholic acid, N-[(3α,5β,12α)-3,12-Dihydroxy-7,24-dioxocholan-24-yl]glycine, uric acid and -

Biliverdin Reductase: a Major Physiologic Cytoprotectant
Biliverdin reductase: A major physiologic cytoprotectant David E. Baran˜ ano*, Mahil Rao*, Christopher D. Ferris†, and Solomon H. Snyder*‡§¶ Departments of *Neuroscience, ‡Pharmacology and Molecular Sciences, and §Psychiatry and Behavioral Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD 21205; and †Department of Medicine, Division of Gastroenterology, C-2104 Medical Center North, Vanderbilt University Medical Center, Nashville, TN 37232-2279 Contributed by Solomon H. Snyder, October 16, 2002 Bilirubin, an abundant pigment that causes jaundice, has long hypothesize that bilirubin acts in a catalytic fashion whereby lacked any clear physiologic role. It arises from enzymatic reduction bilirubin oxidized to biliverdin is rapidly reduced back to bili- by biliverdin reductase of biliverdin, a product of heme oxygenase rubin, a process that could readily afford 10,000-fold amplifica- activity. Bilirubin is a potent antioxidant that we show can protect tion (13). Here we establish that a redox cycle based on BVRA cells from a 10,000-fold excess of H2O2. We report that bilirubin is activity provides physiologic cytoprotection as BVRA depletion a major physiologic antioxidant cytoprotectant. Thus, cellular de- exacerbates the formation of reactive oxygen species (ROS) and pletion of bilirubin by RNA interference markedly augments tissue augments cell death. levels of reactive oxygen species and causes apoptotic cell death. Depletion of glutathione, generally regarded as a physiologic Methods antioxidant cytoprotectant, elicits lesser increases in reactive ox- All chemicals were obtained from Sigma unless otherwise ygen species and cell death. The potent physiologic antioxidant indicated. actions of bilirubin reflect an amplification cycle whereby bilirubin, acting as an antioxidant, is itself oxidized to biliverdin and then Cell Culture and Viability Measurements. -

The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- Or Pro-Oxidant Effect
International Journal of Molecular Sciences Article The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect Annalisa Bianco 1,2, Aleš Dvoˇrák 3 , Nikola Capková 3, Camille Gironde 4, Claudio Tiribelli 1 , Christophe Furger 4, Libor Vitek 3,5 and Cristina Bellarosa 1,* 1 Italian Liver Foundation (FIF), Bldg Q—AREA Science Park Basovizza, SS14 Km 163,5, 34149 Trieste, Italy; [email protected] (A.B.); [email protected] (C.T.) 2 Department of Life Sciences, University of Trieste, 34127 Trieste, Italy 3 Institute of Medical Biochemistry and Laboratory Diagnostics, Faculty General Hospital and 1st Faculty of Medicine, Charles University, 121 08 Prague 2, Czech Republic; [email protected] (A.D.); [email protected] (N.C.); [email protected] (L.V.) 4 AOP/MH2F Team, LAAS-CNRS, 7 avenue de l’Europe, 31400 Toulouse, France; [email protected] (C.G.); [email protected] (C.F.) 5 4th Department of Internal Medicine, Faculty General Hospital and 1st Faculty of Medicine, Charles University, 121 08 Prague 2, Czech Republic * Correspondence: [email protected] Received: 24 September 2020; Accepted: 28 October 2020; Published: 30 October 2020 Abstract: Background: Severe hyperbilirubinemia can cause permanent neurological damage in particular in neonates, whereas mildly elevated serum bilirubin protects from various oxidative stress-mediated diseases. The present work aimed to establish the intracellular unconjugated bilirubin concentrations (iUCB) thresholds differentiating between anti- and pro-oxidant effects. Methods: Hepatic (HepG2), heart endothelial (H5V), kidney tubular (HK2) and neuronal (SH-SY5Y) cell lines were exposed to increasing concentration of bilirubin. iUCB, cytotoxicity, intracellular reactive oxygen species (ROS) concentrations, and antioxidant capacity (50% efficacy concentration (EC50)) were determined. -

©Ferrata Storti Foundation
Original Articles T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response Peter Van Loo,1,2,3 Thomas Tousseyn,4 Vera Vanhentenrijk,4 Daan Dierickx,5 Agnieszka Malecka,6 Isabelle Vanden Bempt,4 Gregor Verhoef,5 Jan Delabie,6 Peter Marynen,1,2 Patrick Matthys,7 and Chris De Wolf-Peeters4 1Department of Molecular and Developmental Genetics, VIB, Leuven, Belgium; 2Department of Human Genetics, K.U.Leuven, Leuven, Belgium; 3Bioinformatics Group, Department of Electrical Engineering, K.U.Leuven, Leuven, Belgium; 4Department of Pathology, University Hospitals K.U.Leuven, Leuven, Belgium; 5Department of Hematology, University Hospitals K.U.Leuven, Leuven, Belgium; 6Department of Pathology, The Norwegian Radium Hospital, University of Oslo, Oslo, Norway, and 7Department of Microbiology and Immunology, Rega Institute for Medical Research, K.U.Leuven, Leuven, Belgium Citation: Van Loo P, Tousseyn T, Vanhentenrijk V, Dierickx D, Malecka A, Vanden Bempt I, Verhoef G, Delabie J, Marynen P, Matthys P, and De Wolf-Peeters C. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolero- genic host immune response. Haematologica. 2010;95:440-448. doi:10.3324/haematol.2009.009647 The Online Supplementary Tables S1-5 are in separate PDF files Supplementary Design and Methods One microgram of total RNA was reverse transcribed using random primers and SuperScript II (Invitrogen, Merelbeke, Validation of microarray results by real-time quantitative Belgium), as recommended by the manufacturer. Relative reverse transcriptase polymerase chain reaction quantification was subsequently performed using the compar- Ten genes measured by microarray gene expression profil- ative CT method (see User Bulletin #2: Relative Quantitation ing were validated by real-time quantitative reverse transcrip- of Gene Expression, Applied Biosystems). -

Bacterial Heme Biosynthesis and Its Biotechnological Application
Appl Microbiol Biotechnol (2003) 63: 115–127 DOI 10.1007/s00253-003-1432-2 MINI-REVIEW N. Frankenberg . J. Moser . D. Jahn Bacterial heme biosynthesis and its biotechnological application Received: 23 June 2003 / Revised: 22 August 2003 / Accepted: 26 July 2003 / Published online: 16 September 2003 # Springer-Verlag 2003 Abstract Proteins carrying a prosthetic heme group are The nickel-containing yellow coenzyme F430 is the vital parts of bacterial energy conserving and stress prosthetic group of methyl-coenzyme M reductase that response systems. They also mediate complex enzymatic catalyzes the final step of methane formation in methano- reactions and regulatory processes. Here, we review the genenic archaea (Thauer and Bonacker 1994). The pink multistep biosynthetic pathway of heme formation includ- cobalt-containing vitamin B12 derivatives are the most ing the enzymes involved and reaction mechanisms. complex known tetrapyrroles (Martens et al. 2002). They Potential biotechnological implications are discussed. are involved in multiple enzymatic reactions, e.g., radical- dependent nucleotide reduction and methyl transfer. The iron-chelating yellow-greenish siroheme is required for the Introduction six electron transfer reactions during assimilatory nitrite or sulfite reduction (Raux et al. 2003). The green pigment Structure and function of tetrapyrroles heme d1, is part of the dissimilatory nitrite reductase in Pseudomonads and is a typical porphinoid that differs Compounds of the tetrapyrrole class are characterized by significantly in structure and color from the other their four five-membered pyrrole rings, usually linked porphyrin-based hemes (Chang 1994). together via single atom bridges (Fig. 1). The four rings of The other commonly found tetrapyrrole structures are the macrocycle are labelled clockwise A–D starting with the open-chain molecules that are all derived from cleaved the first of the three symmetric rings with regard to the macrocycles.