Crystal Structure of Biliverdin Reductase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spectroscopy of Porphyrins
BORIS F. KIM and JOSEPH BOHANDY SPECTROSCOPY OF PORPHYRINS Porphyrins are an important class of compounds that are of interest in molecular biology because of the important roles they play in vital biochemical systems such as biochemical energy conversion in animals, oxygen transport in blood, and photosynthetic energy conversion in plants. We are studying the physical properties of the energy states of porphyrins using the techniques of ex perimental and theoretical spectroscopy with the aim of contributing to a basic understanding of their biochemical behavior. INTRODUCTION Metalloporphin Porphyrins are a class of complex organic chemical compounds found in such diverse places as crude oil, plants, and human beings. They are, in most cases, tailored to carry out vital chemical transformations in intricate biochemical or biophysical systems. They are the key constituents of chlorophyll in plants and of hemoglobin in animals. Without them, life would y be impossible. t Free base porphin These molecules display a wide range of chemical and physical properties that depend on the structural details of the particular porphyrin molecule. All por ~x phyrins are vividly colored and absorb light in the visible and ultraviolet regions of the spectrum. Some exhibit luminescence, paramagnetism, photoconduc tion, or semiconduction. Spme are photosensitizers Wavelength (nanometers) or catalysts. Scientists from several disciplines have been interested in unraveling the principles that cause Fig. 1-The chemical structures for the two forms of por· this diversity of properties. phin are shown on the left. A carbon atom and a hydrogen The simplest compound of all porphyrins is por atom are understood to be at each apex not attached to a nitrogen atom. -

Amplitaq and Amplitaq Gold DNA Polymerase
AmpliTaq and AmpliTaq Gold DNA Polymerase The Most Referenced Brand of DNA Polymerase in the World Date: 2005-05 Notes: Authors are listed alphabetically J. Biol. Chem. (223) Ezoe, S., I. Matsumura, et al. (2005). "GATA Transcription Factors Inhibit Cytokine-dependent Growth and Survival of a Hematopoietic Cell Line through the Inhibition of STAT3 Activity." J. Biol. Chem. 280(13): 13163-13170. http://www.jbc.org/cgi/content/abstract/280/13/13163 Although GATA-1 and GATA-2 were shown to be essential for the development of hematopoietic cells by gene targeting experiments, they were also reported to inhibit the growth of hematopoietic cells. Therefore, in this study, we examined the effects of GATA-1 and GATA-2 on cytokine signals. A tamoxifen-inducible form of GATA-1 (GATA-1/ERT) showed a minor inhibitory effect on interleukin-3 (IL-3)-dependent growth of an IL-3-dependent cell line Ba/F3. On the other hand, it drastically inhibited TPO-dependent growth and gp130-mediated growth/survival of Ba/F3. Similarly, an estradiol-inducible form of GATA-2 (GATA-2/ER) disrupted thrombopoietin (TPO)-dependent growth and gp130-mediated growth/survival of Ba/F3. As for this mechanism, we found that both GATA-1 and GATA-2 directly bound to STAT3 both in vitro and in vivo and inhibited its DNA-binding activity in gel shift assays and chromatin immunoprecipitation assays, whereas they hardly affected STAT5 activity. In addition, endogenous GATA-1 was found to interact with STAT3 in normal megakaryocytes, suggesting that GATA-1 may inhibit STAT3 activity in normal hematopoietic cells. -

Electronic Spectroscopy of Free Base Porphyrins and Metalloporphyrins
Absorption and Fluorescence Spectroscopy of Tetraphenylporphyrin§ and Metallo-Tetraphenylporphyrin Introduction The word porphyrin is derived from the Greek porphura meaning purple, and all porphyrins are intensely coloured1. Porphyrins comprise an important class of molecules that serve nature in a variety of ways. The Metalloporphyrin ring is found in a variety of important biological system where it is the active component of the system or in some ways intimately connected with the activity of the system. Many of these porphyrins synthesized are the basic structure of biological porphyrins which are the active sites of numerous proteins, whose functions range from oxygen transfer and storage (hemoglobin and myoglobin) to electron transfer (cytochrome c, cytochrome oxidase) to energy conversion (chlorophyll). They also have been proven to be efficient sensitizers and catalyst in a number of chemical and photochemical processes especially photodynamic therapy (PDT). The diversity of their functions is due in part to the variety of metals that bind in the “pocket” of the porphyrin ring system (Fig. 1). Figure 1. Metallated Tetraphenylporphyrin Upon metalation the porphyrin ring system deprotonates, forming a dianionic ligand (Fig. 2). The metal ions behave as Lewis acids, accepting lone pairs of electrons ________________________________ § We all need to thank Jay Stephens for synthesizing the H2TPP 2 from the dianionic porphyrin ligand. Unlike most transition metal complexes, their color is due to absorption(s) within the porphyrin ligand involving the excitation of electrons from π to π* porphyrin ring orbitals. Figure 2. Synthesis of Zn(TPP) The electronic absorption spectrum of a typical porphyrin consists of a strong transition to the second excited state (S0 S2) at about 400 nm (the Soret or B band) and a weak transition to the first excited state (S0 S1) at about 550 nm (the Q band). -

CYP2A6) by P53
Transcriptional Regulation of Human Stress Responsive Cytochrome P450 2A6 (CYP2A6) by p53 Hao Hu M.Biotech. (Biotechnology) 2012 The University of Queensland B.B.A. 2009 University of Electronic Science and Technology of China B.Sc. (Pharmacy) 2009 University of Electronic Science and Technology of China A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Medicine ABSTRACT Human cytochrome P450 (CYP) 2A6 is highly expressed in the liver and the encoding gene is regulated by various stress activated transcription factors, such as the nuclear factor (erythroid-derived 2)-like 2 (Nrf-2). Unlike the other xenobiotic metabolising CYP enzymes (XMEs), CYP2A6 only plays a minor role in xenobiotic metabolism. The CYP2A6 is highly induced by multiple forms of cellular stress conditions, where XMEs expression is normally inhibited. Recent findings suggest that the CYP2A6 plays an important role in regulating BR homeostasis. A computer based sequence analysis on the 3 kb proximate CYP2A6 promoter revealed several putative binding sites for p53, a protein that mediates regulation of antioxidant and apoptosis pathways. In this study, the role of p53 in CYP2A6 gene regulation is demonstrated. The site closest to transcription start site (TSS) is highly homologous with the p53 consensus sequence. The p53 responsiveness of this site was confirmed by transfections with various stepwise deleted of CYP2A6-5’-Luc constructs containing the putative p53RE. Deletion of the putative p53RE resulted in a total abolishment of p53 responsiveness of CYP2A6 promoter. Specific binding of p53 to the putative p53RE was detected by electrophoresis mobility shift assay. -

Characterisation of Bilirubin Metabolic Pathway in Hepatic Mitochondria Siti Nur Fadzilah Muhsain M.Sc
Characterisation of Bilirubin Metabolic Pathway in Hepatic Mitochondria Siti Nur Fadzilah Muhsain M.Sc. (Medical Research) 2005 Universiti Sains Malaysia Postgrad. Dip. (Toxicology) 2003 University of Surrey B.Sc.(Biomed. Sc.) 2000 Universiti Putra Malaysia A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2014 School of Medicine ABSTRACT Bilirubin (BR), a toxic waste product of degraded haem, is a potent antioxidant at physiological concentrations. To achieve the maximum benefit of BR, its intracellular level needs to be carefully regulated. A system comprising of two enzymes, haem oxygenase-1 (HMOX1) and cytochrome P450 2A5 (CYP2A5) exists in the endoplasmic reticulum (ER), responsible for regulating BR homeostasis. This system is induced in response to oxidative stress. In this thesis, oxidative stress caused accumulation of these enzymes in mitochondria — major producers and targets of reactive oxygen species (ROS) — is demonstrated. To understand the significance of this intracellular targeting, properties of microsomal and mitochondrial BR metabolising enzymes were compared and the capacity of mitochondrial CYP2A5 to oxidise BR in response to oxidative stress is reported. Microsomes and mitochondrial fractions were isolated from liver homogenates of DBA/2J mice, administered with sub-toxic dose of pyrazole, an oxidant stressor. The purity of extracted organelles was determined by analysing the expressions and activities of their respective marker enzymes. HMOX1 and CYP2A5 were significantly increased in microsomes and even more so in mitochondria in response to pyrazole-induced oxidative stress. By contrast, the treatment did not increase either microsomes or mitochondrial Uridine-diphosphate-glucuronosyltransferase 1A1 (UGT1A1), the sole enzyme that catalyses BR elimination through glucuronidation. -

Porphyrins & Bile Pigments
Bio. 2. ASPU. Lectu.6. Prof. Dr. F. ALQuobaili Porphyrins & Bile Pigments • Biomedical Importance These topics are closely related, because heme is synthesized from porphyrins and iron, and the products of degradation of heme are the bile pigments and iron. Knowledge of the biochemistry of the porphyrins and of heme is basic to understanding the varied functions of hemoproteins in the body. The porphyrias are a group of diseases caused by abnormalities in the pathway of biosynthesis of the various porphyrins. A much more prevalent clinical condition is jaundice, due to elevation of bilirubin in the plasma, due to overproduction of bilirubin or to failure of its excretion and is seen in numerous diseases ranging from hemolytic anemias to viral hepatitis and to cancer of the pancreas. • Metalloporphyrins & Hemoproteins Are Important in Nature Porphyrins are cyclic compounds formed by the linkage of four pyrrole rings through methyne (==HC—) bridges. A characteristic property of the porphyrins is the formation of complexes with metal ions bound to the nitrogen atom of the pyrrole rings. Examples are the iron porphyrins such as heme of hemoglobin and the magnesium‐containing porphyrin chlorophyll, the photosynthetic pigment of plants. • Natural Porphyrins Have Substituent Side Chains on the Porphin Nucleus The porphyrins found in nature are compounds in which various side chains are substituted for the eight hydrogen atoms numbered in the porphyrin nucleus. As a simple means of showing these substitutions, Fischer proposed a shorthand formula in which the methyne bridges are omitted and a porphyrin with this type of asymmetric substitution is classified as a type III porphyrin. -

Heme Oxygenase-1 Regulates Mitochondrial Quality Control in the Heart
RESEARCH ARTICLE Heme oxygenase-1 regulates mitochondrial quality control in the heart Travis D. Hull,1,2 Ravindra Boddu,1,3 Lingling Guo,2,3 Cornelia C. Tisher,1,3 Amie M. Traylor,1,3 Bindiya Patel,1,4 Reny Joseph,1,3 Sumanth D. Prabhu,1,4,5 Hagir B. Suliman,6 Claude A. Piantadosi,7 Anupam Agarwal,1,3,5 and James F. George2,3,4 1Department of Medicine, 2Department of Surgery, 3Nephrology Research and Training Center, and 4Comprehensive Cardiovascular Center, University of Alabama at Birmingham, Birmingham, Alabama, USA. 5Department of Veterans Affairs, Birmingham, Alabama, USA. 6Department of Anesthesiology and 7Department of Pulmonary, Allergy and Critical Care, Duke University School of Medicine, Durham, North Carolina, USA. The cardioprotective inducible enzyme heme oxygenase-1 (HO-1) degrades prooxidant heme into equimolar quantities of carbon monoxide, biliverdin, and iron. We hypothesized that HO-1 mediates cardiac protection, at least in part, by regulating mitochondrial quality control. We treated WT and HO-1 transgenic mice with the known mitochondrial toxin, doxorubicin (DOX). Relative to WT mice, mice globally overexpressing human HO-1 were protected from DOX-induced dilated cardiomyopathy, cardiac cytoarchitectural derangement, and infiltration of CD11b+ mononuclear phagocytes. Cardiac-specific overexpression of HO-1 ameliorated DOX-mediated dilation of the sarcoplasmic reticulum as well as mitochondrial disorganization in the form of mitochondrial fragmentation and increased numbers of damaged mitochondria in autophagic vacuoles. HO-1 overexpression promotes mitochondrial biogenesis by upregulating protein expression of NRF1, PGC1α, and TFAM, which was inhibited in WT animals treated with DOX. Concomitantly, HO-1 overexpression inhibited the upregulation of the mitochondrial fission mediator Fis1 and resulted in increased expression of the fusion mediators, Mfn1 and Mfn2. -

CDH12 Cadherin 12, Type 2 N-Cadherin 2 RPL5 Ribosomal
5 6 6 5 . 4 2 1 1 1 2 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 A A A A A A A A A A A A A A A A A A A A C C C C C C C C C C C C C C C C C C C C R R R R R R R R R R R R R R R R R R R R B , B B B B B B B B B B B B B B B B B B B , 9 , , , , 4 , , 3 0 , , , , , , , , 6 2 , , 5 , 0 8 6 4 , 7 5 7 0 2 8 9 1 3 3 3 1 1 7 5 0 4 1 4 0 7 1 0 2 0 6 7 8 0 2 5 7 8 0 3 8 5 4 9 0 1 0 8 8 3 5 6 7 4 7 9 5 2 1 1 8 2 2 1 7 9 6 2 1 7 1 1 0 4 5 3 5 8 9 1 0 0 4 2 5 0 8 1 4 1 6 9 0 0 6 3 6 9 1 0 9 0 3 8 1 3 5 6 3 6 0 4 2 6 1 0 1 2 1 9 9 7 9 5 7 1 5 8 9 8 8 2 1 9 9 1 1 1 9 6 9 8 9 7 8 4 5 8 8 6 4 8 1 1 2 8 6 2 7 9 8 3 5 4 3 2 1 7 9 5 3 1 3 2 1 2 9 5 1 1 1 1 1 1 5 9 5 3 2 6 3 4 1 3 1 1 4 1 4 1 7 1 3 4 3 2 7 6 4 2 7 2 1 2 1 5 1 6 3 5 6 1 3 6 4 7 1 6 5 1 1 4 1 6 1 7 6 4 7 e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m -
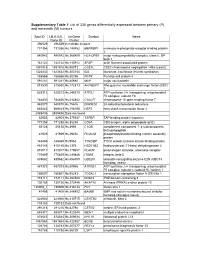
Supplementary Table 1 List of 335 Genes Differentially Expressed Between Primary (P) and Metastatic (M) Tumours
Supplementary Table 1 List of 335 genes differentially expressed between primary (P) and metastatic (M) tumours Spot ID I.M.A.G.E. UniGene Symbol Name Clone ID Cluster 296529 296529 In multiple clusters 731356 731356 Hs.140452 M6PRBP1 mannose-6-phosphate receptor binding protein 1 840942 840942 Hs.368409 HLA-DPB1 major histocompatibility complex, class II, DP beta 1 142122 142122 Hs.115912 AFAP actin filament associated protein 1891918 1891918 Hs.90073 CSE1L CSE1 chromosome segregation 1-like (yeast) 1323432 1323432 Hs.303154 IDS iduronate 2-sulfatase (Hunter syndrome) 788566 788566 Hs.80296 PCP4 Purkinje cell protein 4 591281 591281 Hs.80680 MVP major vault protein 815530 815530 Hs.172813 ARHGEF7 Rho guanine nucleotide exchange factor (GEF) 7 825312 825312 Hs.246310 ATP5J ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F6 784830 784830 Hs.412842 C10orf7 chromosome 10 open reading frame 7 840878 840878 Hs.75616 DHCR24 24-dehydrocholesterol reductase 669443 669443 Hs.158195 HSF2 heat shock transcription factor 2 2485436 2485436 Data not found 82903 82903 Hs.370937 TAPBP TAP binding protein (tapasin) 771258 771258 Hs.85258 CD8A CD8 antigen, alpha polypeptide (p32) 85128 85128 Hs.8986 C1QB complement component 1, q subcomponent, beta polypeptide 41929 41929 Hs.39252 PICALM phosphatidylinositol binding clathrin assembly protein 148469 148469 Hs.9963 TYROBP TYRO protein tyrosine kinase binding protein 415145 415145 Hs.1376 HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 810017 810017 Hs.179657 PLAUR plasminogen activator, -

Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging
biosensors Review Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging Muhammad Imran 1,*, Muhammad Ramzan 2,*, Ahmad Kaleem Qureshi 1, Muhammad Azhar Khan 2 and Muhammad Tariq 3 1 Department of Chemistry, Baghdad-Ul-Jadeed Campus, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan; [email protected] 2 Department of Physics, Baghdad-Ul-Jadeed Campus, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan; [email protected] 3 Institute of Chemical Sciences, Bahauddin Zakariya University, Multan 60800, Pakistan; [email protected] * Correspondence: [email protected] (M.I.); [email protected] (M.R.) Received: 26 September 2018; Accepted: 17 October 2018; Published: 19 October 2018 Abstract: In recent years, scientific advancements have constantly increased at a significant rate in the field of biomedical science. Keeping this in view, the application of porphyrins and metalloporphyrins in the field of biomedical science is gaining substantial importance. Porphyrins are the most widely studied tetrapyrrole-based compounds because of their important roles in vital biological processes. The cavity of porphyrins containing four pyrrolic nitrogens is well suited for the binding majority of metal ions to form metalloporphyrins. Porphyrins and metalloporphyrins possess peculiar photochemical, photophysical, and photoredox properties which are tunable through structural modifications. Their beneficial photophysical properties, such as the long wavelength of emission and absorption, high singlet oxygen quantum yield, and low in vivo toxicity, have drawn scientists’ interest to discover new dimensions in the biomedical field. Applications of porphyrins and metalloporphyrins have been pursued in the perspective of contrast agents for magnetic resonance imaging (MRI), photodynamic therapy (PDT) of cancer, bio-imaging, and other biomedical applications. -

Vitamin B12 Promotes Porphyrin Production in Acne-Associated P
UCLA UCLA Electronic Theses and Dissertations Title Porphyrin production and regulation in different Propionibacterium acnes lineages contribute to acne vulgaris pathogenesis Permalink https://escholarship.org/uc/item/9g02d15m Author Johnson, Tremylla Publication Date 2017 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Porphyrin production and regulation in different Propionibacterium acnes lineages contribute to acne vulgaris pathogenesis A dissertation submitted in partial satisfaction of the requirement for the degree Doctor of Philosophy in Molecular and Medical Pharmacology by Tremylla A. Johnson 2017 © Copyright by Tremylla A. Johnson 2017 ABSTRACT OF THE DISSERTATION Porphyrin production and regulation in different Propionibacterium acnes lineages contribute to acne vulgaris pathogenesis by Tremylla A. Johnson Doctor of Philosophy in Molecular & Medical Pharmacology University of California, Los Angeles, 2017 Professor Huiying Li, Chair Propionibacterium acnes is a dominant human skin commensal. It has been implicated in acne pathogenesis, but its role remains unclear. Recent metagenomic studies have revealed that certain P. acnes strains are highly associated with acne, while some others are associated with healthy skin. Little information exists about P. acnes strain-level differences beyond the genomic differences. In this study, I revealed that acne-associated type IA P. acnes strains produced significantly higher levels of porphyrins (a metabolite important in acne development) than health-associated strains (type II). Strains of type IA-1 and type IA-2 produced similar levels of porphyrins. Moreover, porphyrin production in these P. acnes strains is modulated by vitamin B12. On the other hand, health-associated type II strains produced low levels of ii porphyrins and did not respond to vitamin B12. -

Expanding the Eggshell Colour Gamut: Uroerythrin and Bilirubin from Tinamou (Tinamidae) Eggshells Randy Hamchand1, Daniel Hanley2, Richard O
www.nature.com/scientificreports OPEN Expanding the eggshell colour gamut: uroerythrin and bilirubin from tinamou (Tinamidae) eggshells Randy Hamchand1, Daniel Hanley2, Richard O. Prum3 & Christian Brückner1* To date, only two pigments have been identifed in avian eggshells: rusty-brown protoporphyrin IX and blue-green biliverdin IXα. Most avian eggshell colours can be produced by a mixture of these two tetrapyrrolic pigments. However, tinamou (Tinamidae) eggshells display colours not easily rationalised by combination of these two pigments alone, suggesting the presence of other pigments. Here, through extraction, derivatization, spectroscopy, chromatography, and mass spectrometry, we identify two novel eggshell pigments: yellow–brown tetrapyrrolic bilirubin from the guacamole- green eggshells of Eudromia elegans, and red–orange tripyrrolic uroerythrin from the purplish-brown eggshells of Nothura maculosa. Both pigments are known porphyrin catabolites and are found in the eggshells in conjunction with biliverdin IXα. A colour mixing model using the new pigments and biliverdin reproduces the respective eggshell colours. These discoveries expand our understanding of how eggshell colour diversity is achieved. We suggest that the ability of these pigments to photo- degrade may have an adaptive value for the tinamous. Birds’ eggs are found in an expansive variety of shapes, sizes, and colourings 1. Te diverse array of appearances found across Aves is achieved—in large part—through a combination of structural features, solid or patterned colorations, the use of two diferent dyes, and diferential pigment deposition. Eggshell pigments are embedded within the white calcium carbonate matrix of the egg and within a thin outer proteinaceous layer called the cuticle2–4. Tese pigments are believed to play a key role in crypsis5,6, although other, possibly dynamic 7,8, roles in inter- and intra-species signalling5,9–12 are also possible.