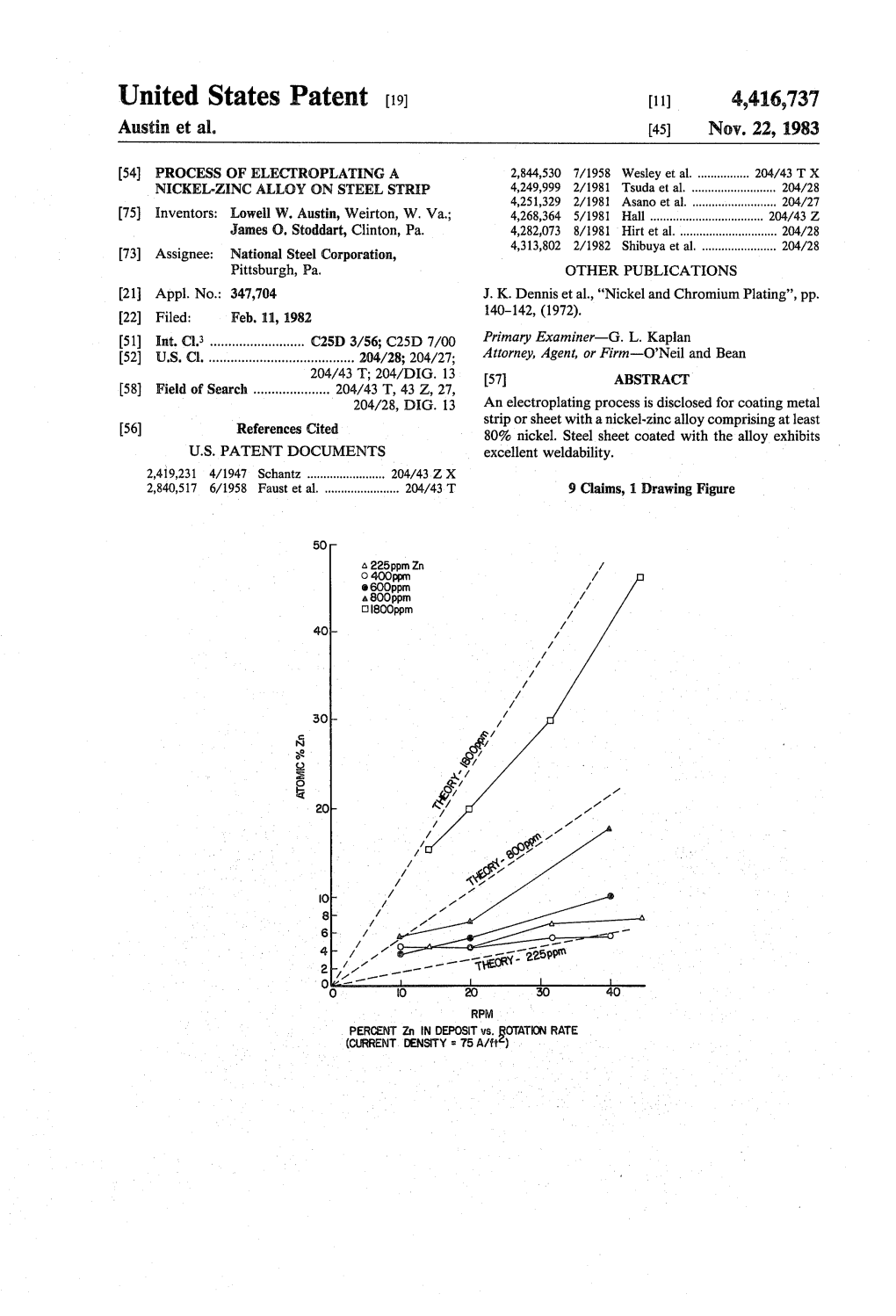
United States Patent (19) 11) 4,416,737 Austin Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016 Summer NRA Program
2016 Summer NRA Program NRA Program Gunsmithing Program Trinidad State Junior College 600 Prospect St. Trinidad, Colorado 81082 1-800-621-8752 ext. 5541 or 719-846-5541 [email protected] TABLE OF CONTENTS Section _____ Page # President’s Welcome 3 Summer Coordinator’s Welcome 4 General Information 5-7 Gunsmithing Technician Certificate 8 Gunsmithing Fine Arts Certificate 8 NRA Firearms Safety Instructor Certificate 9 Using the GI Bill for courses 10 Shipping of Firearms 10 The NRA Foundation Scholarships 11-12 Bill Prator Gun Range 13 2016 Class Schedule 14-15 2016 Firearms Safety Instructor Schedule 16 Detailed Class Schedule – Description, Instructor Information and Tool Lists Week 1 Pgs. 18-25 Week 2 Pgs. 25-36 Week 3 Pgs. 36-45 Week 4 Pgs. 45-55 Week 5 Pgs. 55-61 Week 6 Pgs. 62-64 Week 7 Pgs. 64-75 Week 8 Pgs. 75-85 Week 9 Pgs. 86-91 Registration Form 92 Map of Campus and Driving Directions 94 What to do in and around Trinidad 95 Hotel, Restaurant and Attraction Information The fine print 99 2 PRESIDENT’S WELCOME Excellence. One word says it all. The Gunsmithing School at Trinidad State Junior College has a long tradition of excellence. But don’t take our word for it. Our affiliation with the National Rifle Association assures students that the TSJC Summer NRA program is high quality. For nine glorious weeks, the finest craftsmen and artists from the Gunsmithing world gather in Southern Colorado to offer hands-on courses to students from all over the nation and the world. -

1. Hand Tools 3. Related Tools 4. Chisels 5. Hammer 6. Saw Terminology 7. Pliers Introduction
1 1. Hand Tools 2. Types 2.1 Hand tools 2.2 Hammer Drill 2.3 Rotary hammer drill 2.4 Cordless drills 2.5 Drill press 2.6 Geared head drill 2.7 Radial arm drill 2.8 Mill drill 3. Related tools 4. Chisels 4.1. Types 4.1.1 Woodworking chisels 4.1.1.1 Lathe tools 4.2 Metalworking chisels 4.2.1 Cold chisel 4.2.2 Hardy chisel 4.3 Stone chisels 4.4 Masonry chisels 4.4.1 Joint chisel 5. Hammer 5.1 Basic design and variations 5.2 The physics of hammering 5.2.1 Hammer as a force amplifier 5.2.2 Effect of the head's mass 5.2.3 Effect of the handle 5.3 War hammers 5.4 Symbolic hammers 6. Saw terminology 6.1 Types of saws 6.1.1 Hand saws 6.1.2. Back saws 6.1.3 Mechanically powered saws 6.1.4. Circular blade saws 6.1.5. Reciprocating blade saws 6.1.6..Continuous band 6.2. Types of saw blades and the cuts they make 6.3. Materials used for saws 7. Pliers Introduction 7.1. Design 7.2.Common types 7.2.1 Gripping pliers (used to improve grip) 7.2 2.Cutting pliers (used to sever or pinch off) 2 7.2.3 Crimping pliers 7.2.4 Rotational pliers 8. Common wrenches / spanners 8.1 Other general wrenches / spanners 8.2. Spe cialized wrenches / spanners 8.3. Spanners in popular culture 9. Hacksaw, surface plate, surface gauge, , vee-block, files 10. -

Install Your Gilpin Products Steel Fence
INSTALL YOUR GILPIN PRODUCTS STEEL FENCE Tool You May Need Level Tape Measure Drill Mitre Saw 3/8" Box or socket wrench Flat Screwdriver Center Punch Hammer Scriber or Pencil 1. Install posts. a. Location of posts determines placement and location of fence panels. b. In-ground posts should have at least 24” buried. c. If attaching to concrete, follow directions provided with masonry anchors. To guard against breaking or chipping, install flanges 3”- 4” from edge. d. Posts must be plumb and aligned. (If surface mount posts are not plumb, use shims as needed.) 2. If necessary, cut fence to fit between posts. Insure equal space between post and first picket at either end of panel. 3. Attach fence panels to posts using P/N 865 Adjustable Fittings. a. Drill 1/4” diameter holes approximately 7/8” from each end of both top and bottom fence rails. Mark location using template provided with fitting set. b. Drill 3/16” pilot holes in newel post for self-tapping screws. Use fence to determine location. c. Four inch (4”) space under bottom of pickets is recommended for best appearance. d. Attach L-shaped bracket to fence panel with nuts and bolts provided. e. Attach fence to post using self-tapping screws provided. 4. Tighten all connections. 5. Fence has baked on finish. Touch up hardware, scratches and cuts with rust resistant (not latex) paint. DECO TRITON OASIS (800) 348-0746 www.gilpininc.com [email protected] ©Gilpin Products, LLC. 2019 1 INSTALL YOUR GILPIN PRODUCTS STEEL FENCE Tools you may need Level Tape Measure Drill Hacksaw 3/8” Box or Socket Flat Screwdriver Center Punch Hammer Scriber or Pencil 1. -

An Evaluation of Void Formation in Ex-Service and Creep Tested HP Alloy Tubing Used for Hydrocarbon Reforming
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2014 An Evaluation of Void Formation in Ex-Service and Creep Tested HP Alloy Tubing Used for Hydrocarbon Reforming Zane William Fox Palmer University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Metallurgy Commons Recommended Citation Palmer, Zane William Fox, "An Evaluation of Void Formation in Ex-Service and Creep Tested HP Alloy Tubing Used for Hydrocarbon Reforming. " Master's Thesis, University of Tennessee, 2014. https://trace.tennessee.edu/utk_gradthes/2874 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Zane William Fox Palmer entitled "An Evaluation of Void Formation in Ex-Service and Creep Tested HP Alloy Tubing Used for Hydrocarbon Reforming." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Materials Science and Engineering. Carl D. Lundin, Major Professor We have read this thesis and recommend its acceptance: Hahn Choo, Easo P. George Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) An Evaluation of Void Formation in Ex-Service and Creep Tested HP Alloy Tubing Used for Hydrocarbon Reforming A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Zane William Fox Palmer August 2014 Abstract HP alloy tubing is commonly used in petroleum reforming facilities for its high temperature strength and resistance to corrosion. -

Marking out -Metal
MARKING OUT - METAL ENGINEERS’ SQUARE SCRIBER Before cutting, shaping, drilling or just about doing anything to a piece of sheet metal, you should clearly mark out guidelines to help you work accurately. DIVIDERS There is a whole range of tools designed specifically to help you mark out your metal more easily. You will need to know how to describe these tools for the CENTRE PUNCH exam. Learn their names, their individual parts and learn how to describe how they are used. Click on the black squares above to find out more about the marking out tools used in metalwork. ODD LEG CALIPERS Brannock High School – Technology Department CRAFT & DESIGN SCRIBER WHAT IS IT ? A scriber – this one is double ended although they can be single ended. WHAT IS IT USED FOR ? It is basically used as a pencil when marking out in metalwork. If a pencil or pen was used to mark out in metalwork, the lines would easily rub off. The scriber scores a more permanent line on the surface of the metal which is easier to work with. Brannock High School – Technology Department CRAFT & DESIGN ENGINEERS` SQUARE WHAT IS IT ? An engineers` square is a metalwork tool used to mark out lines at right angles to an edge on metal. WHAT IS IT USED FOR ? The square is pushed against a straight side of the material (e.g. steel). An engineers scriber is then used to scratch a line onto the surface of the metal at right angles to the edge. Sometimes engineers blue (a dye/ink) is wiped onto the surface first so that the scratched line can be seen easily. -

Welding ABSTRACT Units Are General Safety, Basic Metalworking Tools, Layout, Bench Metal Casting, Welding, Metal Finishing, Plan
DOCUMENT RESUME ED 223 837 CE 034 374 TITLE lndustrial Arts Curriculum Guide in Basic Metals. Bulletin No. 1685. INSTITUTION Louisiana State Dept. of Education, Baton Rouge. Div. of Vocational Education. PUB DATE Sep 82 NOTE 127p.; For related documents, see CE 034 372-375. PUB TYPE Guides Classroom Use Guides (For Teachers) (052) EDRS PRICE MF01/PC06 Plus Postage. DESCRIPTORS Behavioral Objectives; *Course Content; Curriculum Guides; Equipment Utilization; Hand Tools; *Industrial Arts; Instructional Materials; Learning Activities; Machine Tools; Metal Industry; *Metals; *Metal Working; Planning; *Program Implementation; Safety; Secondary Education; Sheet Metal Wolk; *Trade and Industrial Education; Vocational Education; Welding IDENTIFIERS *Louisiana ABSTRACT This curriculum guide contains operational guidelines to help local-administrators, teacher educators, and industrial arts teachers in the State of Louisiana determine the extent to which their basic metals courses are meeting the needs of the youth they serve. It consists of a discussion of course prerequisites, goals, content, and implementation as well as 16 units devoted to various subject areas addressed in a basic metals course. Covered in the units are general safety, basic metalworking tools, layout, bench metalwork, sheet metal, art metal, ornamental metalwork, forging, metal casting, welding, metal finishing, planning, careers in metalworking, and basic metals projects. Each unit contains some or all of the following: objectives, time allotments, suggested topics, student activities, teacher activities, resources, and a unit inventory listing necessary tools and equipment. Among those items appended to the guide are safety rules, steps in making a layout, samples of basic metals projects, a sample student-planning sheet, suggestions for measuring achievement, sample test questions, techniques for conducting classes and for motivating students, and a list of resource materials. -

Metalwork KEY WORDS METALWORK KEYWORDS Keywords with Pictures to Trigger Your Memory
Metalwork KEY WORDS METALWORK KEYWORDS Keywords with pictures to trigger your memory. A Revision tool for Junior Certificate Metalwork. Published by Professional Development Service for Teachers (PDST) Junior Certificate School Programme Blackrock Education Centre, Kill Avenue, Dún Laoghaire, Co. Dublin Phone: 01 2365000 Email: [email protected] First Published 2016 Copyright ©PDST, JCSP. The Professional Development Service for Teachers (PDST), The Junior Certificate School Programme (JCSP), the Literacy and Numeracy Strategy, the Demonstration Library Project and the Delivering Equality of Opportunity in Schools (DEIS) Action Plan are funded by the Teacher Education Section of the Department of Education and Skills (DES). All rights reserved. The purpose of this publication is to enhance teaching and learning within the Junior Certificate School Programme. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means for commercial gain without prior written permission of the publisher. Every effort has been made to ensure that this book contains accurate information. However, PDST, JCSP, its agents and the authors shall not be liable for any loss or damage suffered by readers as a result of any information contained herein. Author: Alma Ui Fhlannchadha Scoil Phobail Mhic Dara, Carna, Co.na Gaillimhe 1 NATURES MATERIAL PERIODIC TABLE OF ELEMENTS ELEMENTS MATERIAL Iron (Fe) Steel Carbon (C) Brass Copper (Cu) PVC 2 NATURES MATERIAL States Solid Liquid Gas LIQUID SOLID GAS Water Ice Steam -

ORNAMENTAL IRONWORK Millwork Contracts Our Steam Heated Plant •N»Bl©» Ua to Majiufaoturo Veneer Work at Any
|S e 5 1 & v.; THE ONLY COMMERCIAL NEWSPAPER IN BRITISH COLUMBIA ''A 1 •r. n &** A NBWBPAPSE DEVOTED TO OEN£SAI JfBWS, BVXXtSZNa. CONTBAOTEHT*, ECTQXZTEXaxJrO), FBOTXWOXAX, CITY AND BABBOB X&EPKOVXKEITTS. PubUabed Monday y Offlo* and Plant PBICB—Per Yaar In Advance - % 10.SS- ti& VOL. XVI, No. 73. Wednesday and Friday VANCOUVER, B:C.,$l0imW, JUNE af.1919 ,683 Komer-Xilonafda I>an« Var Month - - - - - ljM Building Material PACIFIC SHEET METAL DOMINION WORKS, Limited Pressed R (J | (^ 1^ Common ROOFING CONTRACTORS KETAL WINDOWS riBE DOORS SKYXXOHTS ^> JOBBINQ BLOW PIPINO SMOKE STACKS Gabriola Shale Products, Limited 1 Wm. N. 0' Co., Li Granville Is. Seymour 2172 Vancouver Office Phone Sey. 8057 Tel. Sey. 4795-6. 548-550 Seymour St. 425 Standard Bank Bldg. J.T.A. Ritchie, Representative It UII.DINO PSBMXT8 AMOVMTMO TO tSOO OB OVEB ISSUED AT TES VAJfCOTTVXB CITT BALL TBBT1BDAT 1 ..... •" - ..:' ;.- . .'_ - -..'.- Number Daacrtpttoa Coat Gtreat Addraaa Xot and Block Subdivision f robJt^ot nort-Ttd* » 7l1-<wn Owmr Addraaa MUNDY, ROWLAND & CO. " - - • ; ' .- - • • • '••• •-,-• • •• " ELECTRICAL ENGINEERS AND f . '•• ' ' • , . CONTRACTORS —— • —-.... — - - I. _ - — i'.''V:.r,.T'1. ° Roofing, Building Clayburn Firebrick, Special PHONE FT.OF COLUMBIA AV Shaped Firebrick, Pressed >M Papers, etc. 2988 Brick, etc. Vancouver Brand Cement, Reinfor Building Partition ced Steel, Hvdrated and Drain Tile Lime, etc. BUILDING AND INDUSTRIAL NEVirs f RANK DARLING & CO. HARD WOO PNEUMATIC TOOLS m Lumber Veneer Panels, Etc. * ••TI-IOR" Our Stock ia tho MostComplete on th* Pacific Coaat TO BUILD SCHOOL ADDITIONS f each, hardwood floors,: built in fixtures^ Ma Beariig Drills -Close Quarter Pistil Air Drills—RrrtttiRC ItMirs Wo aro also Solo Acejits for the Plans are under way in South Van-N* air heat etc., and will copt |3,500 Clippiif Hammers-Wood Binrs-TiriiM aai Electric Drills CeJekrated "Beaver Brand" Maple ea n and Birch Floerlag. -

COPING SAW a Coping Saw Is Used for Cutting Curves and Awkward Shapes in Wood Or Plastic
MARKING OUT TOOLS STEEL RULE This is used to measure a length, draw a straight edge and to test for flatness. It is marked in millimetres (mm) and made in lengths of 150, 300, 500 and 1000mm. 40 50 1mm 5mm 10mm TRY-SQUARE This is used to test that one surface is square (at 90 degrees) to another and for marking out lines square to the face-side or face-edge. Woodworker’s try-squares have a carbon steel blade and a wooden stock, sometimes with a brass face, while engineer’s try-squares have a carbon steel blade and stock. SCRIBER This is used to mark lines on metal and plastic. Hold the scriber like a pencil. When using a scriber make sure that the point is pressed into the angle between the try-square and the material. The point is made of Tool steel and has a 30 degree point angle. COPING SAW A Coping saw is used for cutting curves and awkward shapes in wood or plastic. It has a very thin blade which is kept in tension by the spring metal frame. If the blade breaks a new one can be fitted by unscrewing the handle. The blade of a coping saw can be turned to different positions. This allows you to cut in any direction. HINTS ON USING THE COPING SAW 1 Hold the handle with both hands. 2 The material should be held as low down in the vice as possible. 3 Saw at a slow pace with the blade held horizontal. 4 Always check that the teeth are pointing towards the handle. -

General Workshop
FITTING INTRODUCTION Fitting consists of a handwork involved in fitting together components usually performed at a bench equipped with a vice and hand tools. The matting components have a close relation with each other, and when the function together is termed Fitting. We have to use hand tools, precision tools and various operations, as well as the details of the tool such as identification, material, parts, types, various uses, manipulation, specification, care and maintenance etc. STEEL RULE Blade Stock TRY SQUARE SPRING TYPE OUTSIDE FIRM JOINT OUTSIDE CALIPER CALIPER SAFETY PRECAUTIONS IN FITTING SHOP 1. Shop floor should be kept clean, free from debris, scrap, oil and grease. 2. Do not touch the chip as it comes out of the job. 3. When using grinding machine protect your eyes with goggles. 4. Always work under sufficient light. 5. Do not wear loose dress. 6. Never use hammers with loose heads. 7. Provide guards between opposite vices. 8. Files must have well fitted handles. 9. See that the job is properly fitted to the vice. 10. Do not blow filing when hacksawing. 11. Ease up the pressure when hacksawing is nearly through. 12. Use the right tool for the right job. MEASURING TOOLS AND MARKING TOOLS 1. Steel Rule : A steel rule is a direct reading measuring instrument used to read an accuracy of 0.5mm. Available in various lengths, widths and thickness with several graduations. They are made from high carbon steel, spring steel, stainless steel and various alloy steel. 2. Caliper : It is a simple tool gauging legs. It is made of high carbon steel and the measuring points are hardened and tempered. -

Corrosion of Research Reactor Aluminium Clad Spent Fuel in Water
Technical Reports SeriEs No. 4I8 Corrosion of Research Reactor Aluminium Clad Spent Fuel in Water CORROSION OF RESEARCH REACTOR ALUMINIUM CLAD SPENT FUEL IN WATER The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GREECE PARAGUAY ALBANIA GUATEMALA PERU ALGERIA HAITI PHILIPPINES ANGOLA HOLY SEE POLAND ARGENTINA HONDURAS PORTUGAL ARMENIA HUNGARY QATAR AUSTRALIA ICELAND REPUBLIC OF MOLDOVA AUSTRIA INDIA ROMANIA AZERBAIJAN INDONESIA BANGLADESH IRAN, ISLAMIC REPUBLIC OF RUSSIAN FEDERATION BELARUS IRAQ SAUDI ARABIA BELGIUM IRELAND SENEGAL BENIN ISRAEL SERBIA AND MONTENEGRO BOLIVIA ITALY SEYCHELLES BOSNIA AND JAMAICA SIERRA LEONE HERZEGOVINA JAPAN SINGAPORE BOTSWANA JORDAN SLOVAKIA BRAZIL KAZAKHSTAN SLOVENIA BULGARIA KENYA SOUTH AFRICA BURKINA FASO KOREA, REPUBLIC OF SPAIN CAMEROON KUWAIT SRI LANKA CANADA KYRGYZSTAN SUDAN CENTRAL AFRICAN LATVIA SWEDEN REPUBLIC LEBANON CHILE LIBERIA SWITZERLAND CHINA LIBYAN ARAB JAMAHIRIYA SYRIAN ARAB REPUBLIC COLOMBIA LIECHTENSTEIN TAJIKISTAN COSTA RICA LITHUANIA THAILAND CÔTE D’IVOIRE LUXEMBOURG THE FORMER YUGOSLAV CROATIA MADAGASCAR REPUBLIC OF MACEDONIA CUBA MALAYSIA TUNISIA CYPRUS MALI TURKEY CZECH REPUBLIC MALTA UGANDA DEMOCRATIC REPUBLIC MARSHALL ISLANDS UKRAINE OF THE CONGO MAURITIUS UNITED ARAB EMIRATES DENMARK MEXICO UNITED KINGDOM OF DOMINICAN REPUBLIC MONACO GREAT BRITAIN AND ECUADOR MONGOLIA NORTHERN IRELAND EGYPT MOROCCO UNITED REPUBLIC EL SALVADOR MYANMAR OF TANZANIA ERITREA NAMIBIA UNITED STATES OF AMERICA ESTONIA NETHERLANDS URUGUAY ETHIOPIA NEW ZEALAND FINLAND NICARAGUA UZBEKISTAN FRANCE NIGER VENEZUELA GABON NIGERIA VIETNAM GEORGIA NORWAY YEMEN GERMANY PAKISTAN ZAMBIA GHANA PANAMA ZIMBABWE The Agency’s Statute was approved on 23 October 1956 by the Conference on the Statute of the IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957. -

UNIT 10 BASIC LAYOUT OPERATIONS Operations
Basic Layout UNIT 10 BASIC LAYOUT OPERATIONS Operations Structure 10.1 Introduction Objectives 10.2 Preparing Surface for Layout 10.3 To Layout Parallel Lines to an Edge 10.4 To Layout Lines at Right Angles 10.5 To Layout Horizontal Lines Using a Surface Gauge 10.6 Laying Out Centre Holes 10.7 To Check the Accuracy of the Centre Layout 10.7.1 Divider Method 10.7.2 Lathe Centre Method 10.8 Summary 10.9 Key Words 10.1 INTRODUCTION The preparation of the work surface and laying out straight lines are the primary operations performed in order to make a layout. Since the accuracy of the finished workpiece depends upon the accuracy of the layout, proper care should be taken during the process. Combination tools are used where the accuracy is not important. Square head is used to lay out horizontal and parallel straight lines. The bevel protractor is used for laying out angles while the centre head is used to layout the centre of round and square workpiece. Objectives After studying this unit, you should be able to • know how to prepare the work surface for layout, • layout straight lines using : ¾ the scriber and combination square ¾ the surface gauge and surface plate ¾ the vernier height gauge and surface plate • locate centre holes by : ¾ hermaphrodite calipers ¾ centre head ¾ surface gauge. 10.2 PREPARING SURFACE FOR LAYOUT Layout lines must be visually plain. Therefore, the surface of the work is coated with a layout material so that scribed lines are seen easily. The surface must be clean and free from dust, grease, surface scale, oil, etc., otherwise layout material will not stick to it.