A Systematic Review of the Evidence Supporting a Role for Vasopressor Support in Acute SCI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suplento1 Volumen 71 En
S1 Volumen 71 Mayo 2015 Revista Española de Vol. 71 Supl. 1 • Mayo 2015 Vol. Clínica e Investigación Órgano de expresión de la Sociedad Española de SEINAP Investigación en Nutrición y Alimentación en Pediatría Sumario XXX CONGRESO DE LA SOCIEDAD espaÑOLA DE CUIDADOS INTENSIVOS PEDIÁTRICOS Toledo, 7-9 de mayo de 2015 MESA REDONDA: ¿HACIA DÓNDE VAMOS EN LA MESA REDONDA: EL PACIENTE AGUDO MONITORIZACIÓN? CRONIFICADO EN UCIP 1 Monitorización mediante pulsioximetría: ¿sólo saturación 47 Nutrición en el paciente crítico de larga estancia en UCIP. de oxígeno? P. García Soler Z. Martínez de Compañón Martínez de Marigorta 3 Avances en la monitorización de la sedoanalgesia. S. Mencía 53 Traqueostomía, ¿cuándo realizarla? M.A. García Teresa Bartolomé y Grupo de Sedoanalgesia de la SECIP 60 Los cuidados de enfermería, ¿un reto? J.M. García Piñero 8 Avances en neuromonitorización. B. Cabeza Martín CHARLA-COLOQUIO SESIÓN DE PUESTA AL DÍA: ¿ES BENEFICIOSA LA 64 La formación en la preparación de las UCIPs FLUIDOTERAPIA PARA MI PACIENTE? españolas frente al riesgo de epidemias infecciosas. 13 Sobrecarga de líquidos y morbimortalidad asociada. J.C. de Carlos Vicente M.T. Alonso 68 Lecciones aprendidas durante la crisis del Ébola: 20 Estrategias de fluidoterapia racional en Cuidados experiencia del intensivista de adultos. J.C. Figueira Intensivos Pediátricos. P. de la Oliva Senovilla Iglesias 72 El niño con enfermedad por virus Ébola: un nuevo reto MESA REDONDA: INDICADORES DE CALIDAD para el intensivista pediátrico. E. Álvarez Rojas DE LA SECIP 23 Evolución de la cultura de seguridad en UCIP. MESA REDONDA: UCIP ABIERTAS 24 HORAS, La comunicación efectiva. -

Evaluation and Management of Bradydysrhythmias
VISIT US AT BOOTH # 116 AT THE ACEP SCIENTIFIC ASSEMBLY IN SEATTLE, OCTOBER 14-16, 2013 September 2013 Evaluation And Management Volume 15, Number 9 Of Bradydysrhythmias In The Author Nathan Deal, MD Assistant Professor, Section of Emergency Medicine, Baylor Emergency Department College of Medicine, Houston, TX Peer Reviewers Abstract Joshua M. Kosowsky, MD Vice Chair for Clinical Affairs, Department of Emergency Medicine, Brigham and Women’s Hospital; Assistant Professor, Bradydysrhythmias represent a collection of cardiac conduction Harvard Medical School, Boston, MA abnormalities that span the spectrum of emergency presentations, Charles V. Pollack, Jr., MA, MD, FACEP Professor and Chair, Department of Emergency Medicine, from relatively benign conditions to conditions that represent Pennsylvania Hospital, Perelman School of Medicine, University serious, life-threatening emergencies. This review presents the of Pennsylvania, Philadelphia, PA electrocardiographic findings seen in common bradydysrhythmias CME Objectives and emphasizes prompt recognition of these patterns. Underlying Upon completion of this article, you should be able to: etiologies that may accompany these conduction abnormalities are 1. Recognize the electrocardiographic features of common discussed, including bradydysrhythmias that are reflex mediated bradydysrhythmias. (including trauma induced) and those with metabolic, environ- 2. Consider a variety of pathologies that give rise to mental, infectious, and toxicologic causes. Evidence regarding the bradydysrhythmias. 3. Identify the emergent therapies for the unstable patient management of bradydysrhythmias in the emergency department with bradycardia. is limited; however, there are data to guide the approach to the un- 4. Be familiar with common antidotes for acute toxicities that stable bradycardic patient. When decreased end-organ perfusion is result in bradydysrhythmias. -

Pediatric Shock
REVIEW Pediatric shock Usha Sethuraman† & Pediatric shock accounts for significant mortality and morbidity worldwide, but remains Nirmala Bhaya incompletely understood in many ways, even today. Despite varied etiologies, the end result †Author for correspondence of pediatric shock is a state of energy failure and inadequate supply to meet the metabolic Children’s Hospital of Michigan, Division of demands of the body. Although the mortality rate of septic shock is decreasing, the severity Emergency Medicine, is on the rise. Changing epidemiology due to effective eradication programs has brought in Carman and Ann Adams new microorganisms. In the past, adult criteria had been used for the diagnosis and Department of Pediatrics, 3901 Beaubien Boulevard, management of septic shock in pediatrics. These have been modified in recent times to suit Detroit, MI 48201, USA the pediatric and neonatal population. In this article we review the pathophysiology, Tel.: +1 313 745 5260 epidemiology and recent guidelines in the management of pediatric shock. Fax: +1 313 993 7166 [email protected] Shock is an acute syndrome in which the circu- to generate ATP. It is postulated that in the face of latory system is unable to provide adequate oxy- prolonged systemic inflammatory insult, overpro- gen and nutrients to meet the metabolic duction of cytokines, nitric oxide and other medi- demands of vital organs [1]. Due to the inade- ators, and in the face of hypoxia and tissue quate ATP production to support function, the hypoperfusion, the body responds by turning off cell reverts to anaerobic metabolism, causing the most energy-consuming biophysiological acute energy failure [2]. -
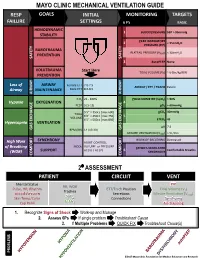
Mechanical Ventilation Guide
MAYO CLINIC MECHANICAL VENTILATION GUIDE RESP GOALS INITIAL MONITORING TARGETS FAILURE SETTINGS 6 P’s BASIC HEMODYNAMIC 1 BLOOD PRESSURE SBP > 90mmHg STABILITY PEAK INSPIRATORY 2 < 35cmH O PRESSURE (PIP) 2 BAROTRAUMA PLATEAU PRESSURE (P ) < 30cmH O PREVENTION PLAT 2 SAFETY SAFETY 3 AutoPEEP None VOLUTRAUMA Start Here TIDAL VOLUME (V ) ~ 6-8cc/kg IBW PREVENTION T Loss of AIRWAY Female ETT 7.0-7.5 AIRWAY / ETT / TRACH Patent Airway MAINTENANCE Male ETT 8.0-8.5 AIRWAY AIRWAY FiO2 21 - 100% PULSE OXIMETRY (SpO2) > 90% Hypoxia OXYGENATION 4 PEEP 5 [5-15] pO2 > 60mmHg 5’5” = 350cc [max 600] pCO2 40mmHg TIDAL 6’0” = 450cc [max 750] 5 VOLUME 6’5” = 500cc [max 850] ETCO2 45 Hypercapnia VENTILATION pH 7.4 GAS GAS EXCHANGE BPM (RR) 14 [10-30] GAS EXCHANGE MINUTE VENTILATION (VMIN) > 5L/min SYNCHRONY WORK OF BREATHING Decreased High Work ASSIST CONTROL MODE VOLUME or PRESSURE of Breathing PATIENT-VENTILATOR AC (V) / AC (P) 6 Comfortable Breaths (WOB) SUPPORT SYNCHRONY COMFORT COMFORT 2⁰ ASSESSMENT PATIENT CIRCUIT VENT Mental Status PIP RR, WOB Pulse, HR, Rhythm ETT/Trach Position Tidal Volume (V ) Trachea T Blood Pressure Secretions Minute Ventilation (V ) SpO MIN Skin Temp/Color 2 Connections Synchrony ETCO Cap Refill 2 Air-Trapping 1. Recognize Signs of Shock Work-up and Manage 2. Assess 6Ps If single problem Troubleshoot Cause 3. If Multiple Problems QUICK FIX Troubleshoot Cause(s) PROBLEMS ©2017 Mayo Clinic Foundation for Medical Education and Research CAUSES QUICK FIX MANAGEMENT Bleeding Hemostasis, Transfuse, Treat cause, Temperature control HYPOVOLEMIA Dehydration Fluid Resuscitation (End points = hypoxia, ↑StO2, ↓PVI) 3rd Spacing Treat cause, Beware of hypoxia (3rd spacing in lungs) Pneumothorax Needle D, Chest tube Abdominal Compartment Syndrome FLUID Treat Cause, Paralyze, Surgery (Open Abdomen) OBSTRUCTED BLOOD RETURN Air-Trapping (AutoPEEP) (if not hypoxic) Pop off vent & SEE SEPARATE CHART PEEP Reduce PEEP Cardiac Tamponade Pericardiocentesis, Drain. -

Approach to Shock.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Approach to Shock.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Approach to Shock Developed by Dr. Dustin Jacobson and Dr Suzanne Beno for PedsCases.com. December 20, 2016 My name is Dustin Jacobson, a 3rd year pediatrics resident from the University of Toronto. This podcast was supervised by Dr. Suzanne Beno, a staff physician in the division of Pediatric Emergency Medicine at the University of Toronto. Today, we’ll discuss an approach to shock in children. First, we’ll define shock and understand it’s pathophysiology. Next, we’ll examine the subclassifications of shock. Last, we’ll review some basic and more advanced treatment for shock But first, let’s start with a case. Jonny is a 6-year-old male who presents with lethargy that is preceded by 2 days of a diarrheal illness. He has not urinated over the previous 24 hours. On assessment, he is tachycardic and hypotensive. He is febrile at 40 degrees Celsius, and is moaning on assessment, but spontaneously breathing. We’ll revisit this case including evaluation and management near the end of this podcast. The term “shock” is essentially a ‘catch-all’ phrase that refers to a state of inadequate oxygen or nutrient delivery for tissue metabolic demand. This broad definition incorporates many causes that eventually lead to this end-stage state. Basic oxygen delivery is determined by cardiac output and content of oxygen in the blood. -

Hypothesis Spinal Shock and `Brain Death': Somatic Pathophysiological
Spinal Cord (1999) 37, 313 ± 324 ã 1999 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/99 $12.00 http://www.stockton-press.co.uk/sc Hypothesis Spinal shock and `brain death': Somatic pathophysiological equivalence and implications for the integrative-unity rationale DA Shewmon*,1 1Pediatric Neurology, UCLA Medical School, Los Angeles, California, USA The somatic pathophysiology of high spinal cord injury (SCI) not only is of interest in itself but also sheds light on one of the several rationales proposed for equating `brain death' (BD) with death, namely that the brain confers integrative unity upon the body, which would otherwise constitute a mere conglomeration of cells and tissues. Insofar as the neuropathology of BD includes infarction down to the foramen magnum, the somatic pathophysiology of BD should resemble that of cervico-medullary junction transection plus vagotomy. The endocrinologic aspects can be made comparable either by focusing on BD patients without diabetes insipidus or by supposing the victim of high SCI to have pre-existing therapeutically compensated diabetes insipidus. The respective literatures on intensive care for BD organ donors and high SCI corroborate that the two conditions are somatically virtually identical. If SCI victims are alive at the level of the `organism as a whole', then so must be BD patients (the only signi®cant dierence being consciousness). Comparison with SCI leads to the conclusion that if BD is to be equated with death, a more coherent reason must be adduced than that the body as a biological organism is dead. Keywords: brain death; spinal cord injury; spinal shock; integrative functions; somatic integrative unity; organism as a whole Introduction Spinal shock is a transient functional depression of the society; its legal de®nition is culturally relative, and structurally intact cord below a lesion, following acute most modern societies happen to have chosen to spinal cord injury (SCI). -

Cardiovascular Responses to Hypoxemia in Sinoaortic-Denervated Fetal Sheep
003 1-399819 1 /3004-038 1$03.0010 PEDIATRIC RESEARCH Vol. 30. No. 4, I991 Copyright ID1991 International Pediatric Research Foundation. Inc. I1riiirc~c/it1 U.S. ,.I Cardiovascular Responses to Hypoxemia in Sinoaortic-Denervated Fetal Sheep JOSEPH ITSKOVITZ (ELDOR), EDMOND F. LAGAMMA. JAMES BRISTOW, AND ABRAHAM M. RUDOLPH Ccirdiovascz~karResearch Instillrle. Unlver:c.i/yqf Califi~rniu,Sari Francisco. Sun Francisco. Cu11fi)rilia94/43 ABSTRACT. Fetal cardiovascular response to acute hy- hypoxemia in postnatal life (1 3). The vascular effects of periph- poxemia is characterized by bradycardia, hypertension, and eral chemoreceptor stimulation, with ventilation held constant, redistribution of cardiac output. The role of aortic and include coronary vasodilation and vasoconstriction in the carotid chemoreceptors in mediating these responses was splanchnic organs and the skeletal muscles. Stimulation of the examined in eight sinoaortic-denervated and nine sham- carotid body chemoreceptors results in reflex bradycardia and operated fetal lambs. Blood gases, pH, heart rate, arterial negative inotropic responses. The bradycardia and peripheral pressure, and blood flow distribution were determined be- vasoconstriction during carotid chemoreceptor stimulation can fore and during hypoxemia. In intact fetuses, heart rate be reversed by effects arising from concurrent hypernea (13). fell from 184 -+ 12 to 165 + 23 beatslmin (p< 0.01) but The arterial chemoreceptors (aortic and carotid bodies) are increased from 184 + 22 to 200 + 16 beatslmin (p< 0.05) active in the fetal lamb and are responsive to hypoxemia (14- in the sinoaortic-denervated fetuses. Intact fetuses showed 21). Stimulation of the fetal arterial chemoreceptors result in an early hypertensive response to hypoxemia, whereas the bradycardia, which is abolished by SAD (19, 20, 22). -

Investigation of the Cardiovascular Action of Sympathetic Amines Using Two Kinds of Strain Gauge Instrument
Nagoya ]. med. Sci. 29: 155-165, 1966. INVESTIGATION OF THE CARDIOVASCULAR ACTION OF SYMPATHETIC AMINES USING TWO KINDS OF STRAIN GAUGE INSTRUMENT ATSUSHI SEKIYA, MITSUYOSHI NAKASHIMA, AND ZENGO KANDA Department of Pharmcology, Nagoya University School of Medicine (Director: Prof. Zen go Kanda) SUMMARY Ventricular responses of a few catecholamines in rabbits were studied with the use of various parameters: blood pressure, systemic output or stroke volume, heart rate, ventricular contractile force and change of segment length of ventricular muscle. In recording ventricular contraction, two kinds of apparatus, strain gauge compass and arch, which we devised, were used. Simultaneous recordings of these various factors permit characterization and direct comparison of the nature and sequence of left ventricular responses by infusion of catecholamines. Epinephrine or norepinephrine, in smaller dose produced almost the same changes in ventricular contractile force and segment length of ventricular muscle. However, in the course of a short time after the administration of the large dose of these drugs, the change of coutractile force by means of strain gauge arch was significant, but the change of muscle segment length measused by means of strain gauge compass was more complicated and gave us much information of cardiac function. Namely, there were a decrease of stroke deflection with a decrease of stroke volume and a downward displacement of systolic and diastolic excursion curve which meant heart dilatation. By the administration of methoxamine, the changes of muscle segment length were more marked than the changes of contractile force. By the administration of isoproterenol, the changes of contractile force were more marked than the changes of muscle segment length changes. -

Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity
REVIEW published: 15 August 2017 doi: 10.3389/fnins.2017.00461 Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms Roger A. L. Dampney* Physiology, School of Medical Sciences, The University of Sydney, Sydney, NSW, Australia The baroreceptor reflex controls arterial pressure primarily via reflex changes in vascular resistance, rather than cardiac output. The vascular resistance in turn is dependent upon the activity of sympathetic vasomotor nerves innervating arterioles in different vascular beds. In this review, the major theme is that the baroreflex control of sympathetic vasomotor activity is not constant, but varies according to the behavioral state of the animal. In contrast to the view that was generally accepted up until the 1980s, I argue that the baroreflex control of sympathetic vasomotor activity is not inhibited or overridden during behaviors such as mental stress or exercise, but instead is reset under those Edited by: Chloe E. Taylor, conditions so that it continues to be highly effective in regulating sympathetic activity and Western Sydney University, Australia arterial blood pressure at levels that are appropriate for the particular ongoing behavior. A Reviewed by: major challenge is to identify the central mechanisms and neural pathways that subserve Satoshi Iwase, Aichi Medical University, Japan such resetting in different states. A model is proposed that is capable of simulating the Craig D. Steinback, different ways in which baroreflex resetting is occurred. Future studies are required to University of Alberta, Canada determine whether this proposed model is an accurate representation of the central *Correspondence: mechanisms responsible for baroreflex resetting. -

SIMULATED HUMAN DIVING and HEART RATE: MAKING the MOST of the DIVING RESPONSE AS a LABORATORY EXERCISE Sara M
SIMULATED HUMAN DIVING AND HEART RATE: MAKING THE MOST OF THE DIVING RESPONSE AS A LABORATORY EXERCISE Sara M. Hiebert and Elliot Burch Advan Physiol Educ 27:130-145, 2003. doi:10.1152/advan.00045.2002 You might find this additional information useful... This article cites 15 articles, 3 of which you can access free at: http://ajpadvan.physiology.org/cgi/content/full/27/3/130#BIBL Medline items on this article's topics can be found at http://highwire.stanford.edu/lists/artbytopic.dtl on the following topics: Pharmacology .. Heart Diseases (Drug Development) Physiology .. Exertion Medicine .. Fitness (Physical Activity) Medicine .. Exercise Medicine .. Bradycardia Downloaded from Physiology .. Humans Updated information and services including high-resolution figures, can be found at: http://ajpadvan.physiology.org/cgi/content/full/27/3/130 Additional material and information about Advances in Physiology Education can be found at: http://www.the-aps.org/publications/advan ajpadvan.physiology.org This information is current as of January 10, 2007 . on January 10, 2007 Advances in Physiology Education is dedicated to the improvement of teaching and learning physiology, both in specialized courses and in the broader context of general biology education. It is published four times a year in March, June, September and December by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2005 by the American Physiological Society. ISSN: 1043-4046, ESSN: 1522-1229. Visit our website at http://www.the-aps.org/. T E A C H I N G I N T H E L A B O R A T O R Y SIMULATED HUMAN DIVING AND HEART RATE: MAKING THE MOST OF THE DIVING RESPONSE AS A LABORATORY EXERCISE Sara M. -

Polytrauma Shock • Trauma Related Costs in the U.S
POLYTRAUMA SHOCK • TRAUMA RELATED COSTS IN THE U.S. 400 BILLION $ • 3.8 MILLION DEATHS PER YEAR • THE LEADING CAUSE OF DEATH IN PERSONS AGED 1 TO 45 IN THE MOST DEVELOPED COUNTRIES • TRIMODAL DEATH DISTRIBUTION 1. SECONDS, MINS FOLLOWING INJURY DUE TO BRAIN, C. SPINE, LARGE VESSEL INJ. 2. 1-2 HOURS FOLLOWING INJURY DUE TO EPIDURAL HAEMATOMAS, BLEEDINGS GOLDEN HOUR 3. SEVERAL DAYS FOLLOWING INJURY DUE TO M.O.F., SEPSIS TRIMODAL DEATH DISTRIBUTION 50% 40% 30% 20% 10% 0% HOURS 0 1 2 3 4 WEEKS 1 2 3 4 TRIMODAL BIMODAL? THE SECOND GROUP IS DECREASING DUE TO PROPER TREATMENT GOLDEN HOUR NOT ONLY SALVAGEABILITY BUT MANY LATE PROBLEMS (SIRS, MOF) ARE THE CONSEQUENCES OF THE PRIMARY HYPOXIA AND MEDIATOR RELEASE SILVER DAY BRONZE WEEK PLATINA 10 MIN DEFINITION OF POLYTRAUMA A. INJURY TO ONE OR MORE BODY REGIONS OR ORGANS OF WHICH ONE, OR THEIR COMBINATIONS IS LIFE THREATENING B. INJURY TO MORE BODY REGIONS FOLLOWING WHICH, DURING TREATMENT, WE HAVE TO MAKE COMPROMISES C. INJURY TO HOLLOW ORGANS + INJURY TO EXTREMITIES D. INJURY DEFINED BY A SCORING SYSTEM TRAUMA SCORE NUMBER OF BREATHS PER MIN 0-4 INTENSITY OF BREATHING 0-1 SYSTOLIC BLOOD PRESSURE 0-4 CAPILLARY REFILL 0-2 GLASGOW COMA SCALE 0-5 POSSIBILITY FOR SURVIVAL 16+ <2 99% 0% GLASGOW COMA SCALE EYE OPENING SPONTANEOUS 4 TO SPEECH 3 TO PAIN 2 NONE 1 BEST MOTOR RESPONSE OBEYS COMMANDS 6 LOCALIZES PAIN 5 NORMAL FLEXION 4 ABNORMAL FLEXION 3 EXTENSION 2 NONE 1 VERBAL RESPONSE ORIENTED 5 CONFUSED CONVERS. 4 WORDS 3 SOUNDS 2 NOTHING 1 ABBREVIATED INJURY SCALE (ISS) 1 MILD 2 MEDIUM 3 SEVERE 4 VERY -

Definition Shock Is a Life-Threatening Condition That Occurs When the Body
Shock Definition Shock is a life-threatening condition that occurs when the body is not getting enough blood flow. This can damage multiple organs. Shock requires immediate medical treatment and can get worse very rapidly. Considerations Major classes of shock include: • Cardiogenic shock (associated with heart problems) • Hypovolemic shock (caused by inadequate blood volume) • Anaphylactic shock (caused by allergic reaction) • Septic shock (associated with infections) • Neurogenic shock (caused by damage to the nervous system) Causes Shock can be caused by any condition that reduces blood flow, including: • Heart problems (such as heart attack or heart failure ) • Low blood volume (as with heavy bleeding or dehydration ) • Changes in blood vessels (as with infection or severe allergic reactions ) Shock is often associated with heavy external or internal bleeding from a serious injury. Spinal injuries can also cause shock. Toxic shock syndrome is an example of a type of shock from an infection. Symptoms A person in shock has extremely low blood pressure. Depending on the specific cause and type of shock, symptoms will include one or more of the following: • Anxiety or agitation • Confusion • Pale, cool, clammy skin • Bluish lips and fingernails • Dizziness , light-headedness, fainting or unconsciousness • Profuse sweating , moist skin • Rapid but weak pulse • Shallow breathing • Chest pain First Aid • Call 911 for immediate medical help. • Check the person's airways, breathing, and circulation. If necessary, begin rescue breathing and CPR. • Even if the person is able to breathe on his or her own, continue to check rate of breathing at least every 5 minutes until help arrives. • If the person is conscious and does NOT have an injury to the head, leg, neck, or spine, place the person in the shock position.