How Many Species Are There in Bermuda?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jacksonville, Florida 1998 Odmds Benthic Community Assessment
JACKSONVILLE, FLORIDA 1998 ODMDS BENTHIC COMMUNITY ASSESSMENT Submitted to U.S. Environmental Protection Agency, Region 4 61 Forsyth St. Atlanta, Georgia 30303 Prepared by Barry A. Vittor & Associates, Inc. 8060 Cottage Hill Rd. Mobile, Alabama 36695 (334) 633-6100 November 1999 TABLE OF CONTENTS LIST OF TABLES ………………………………………….……………………………3 LIST OF FIGURES ……………………..………………………………………………..4 1.0 INTRODUCTION ………..…………………………………………………………..5 2.0 METHODS ………..…………………………………………………………………..5 2.1 Sample Collection And Handling ………………………………………………5 2.2 Macroinfaunal Sample Analysis ……………………………………………….6 3.0 DATA ANALYSIS METHODS ……..………………………………………………6 3.1 Assemblage Analyses ..…………………………………………………………6 3.2 Faunal Similarities ……………………………………………………….…….8 4.0 HABITAT CHARACTERISTICS ……………………………………………….…8 5.0 BENTHIC COMMUNITY CHARACTERIZATION ……………………………..9 5.1 Faunal Composition, Abundance, And Community Structure …………………9 5.2 Numerical Classification Analysis …………………………………………….10 5.3 Taxa Assemblages …………………………………………………………….11 6.0 1995 vs 1998 COMPARISONS ……………………………………………………..11 7.0 SUMMARY ………………………………………………………………………….13 8.0 LITERATURE CITED ……………………………………………………………..16 2 LIST OF TABLES Table 1. Station locations for the Jacksonville, Florida ODMDS, June 1998. Table 2. Sediment data for the Jacksonville, Florida ODMDS, June 1998. Table 3. Summary of abundance of major taxonomic groups for the Jacksonville, Florida ODMDS, June 1998. Table 4. Abundance and distribution of major taxonomic groups at each station for the Jacksonville, Florida ODMDS, June 1998. Table 5. Abundance and distribution of taxa for the Jacksonville, Florida ODMDS, June 1998. Table 6. Percent abundance of dominant taxa (> 5% of the total assemblage) for the Jacksonville, Florida ODMDS, June 1998. Table 7. Summary of assemblage parameters for the Jacksonville, Florida ODMDS stations, June 1998. Table 8. Analysis of variance table for density differences between stations for the Jacksonville, Florida ODMDS stations, June 1998. -

Howard Associate Professor of Natural History and Curator Of
INGI AGNARSSON PH.D. Howard Associate Professor of Natural History and Curator of Invertebrates, Department of Biology, University of Vermont, 109 Carrigan Drive, Burlington, VT 05405-0086 E-mail: [email protected]; Web: http://theridiidae.com/ and http://www.islandbiogeography.org/; Phone: (+1) 802-656-0460 CURRICULUM VITAE SUMMARY PhD: 2004. #Pubs: 138. G-Scholar-H: 42; i10: 103; citations: 6173. New species: 74. Grants: >$2,500,000. PERSONAL Born: Reykjavík, Iceland, 11 January 1971 Citizenship: Icelandic Languages: (speak/read) – Icelandic, English, Spanish; (read) – Danish; (basic) – German PREPARATION University of Akron, Akron, 2007-2008, Postdoctoral researcher. University of British Columbia, Vancouver, 2005-2007, Postdoctoral researcher. George Washington University, Washington DC, 1998-2004, Ph.D. The University of Iceland, Reykjavík, 1992-1995, B.Sc. PROFESSIONAL AFFILIATIONS University of Vermont, Burlington. 2016-present, Associate Professor. University of Vermont, Burlington, 2012-2016, Assistant Professor. University of Puerto Rico, Rio Piedras, 2008-2012, Assistant Professor. National Museum of Natural History, Smithsonian Institution, Washington DC, 2004-2007, 2010- present. Research Associate. Hubei University, Wuhan, China. Adjunct Professor. 2016-present. Icelandic Institute of Natural History, Reykjavík, 1995-1998. Researcher (Icelandic invertebrates). Institute of Biology, University of Iceland, Reykjavík, 1993-1994. Research Assistant (rocky shore ecology). GRANTS Institute of Museum and Library Services (MA-30-19-0642-19), 2019-2021, co-PI ($222,010). Museums for America Award for infrastructure and staff salaries. National Geographic Society (WW-203R-17), 2017-2020, PI ($30,000). Caribbean Caves as biodiversity drivers and natural units for conservation. National Science Foundation (IOS-1656460), 2017-2021: one of four PIs (total award $903,385 thereof $128,259 to UVM). -

Proceedings of the Helminthological Society of Washington 43(2) 1976
Volume July 1976 Number 2 PROCEEDINGS '* " ' "•-' ""' ' - ^ \~ ' '':'-'''' ' - ~ .•' - ' ' '*'' '* ' — "- - '• '' • The Helminthologieal Society of Washington ., , ,; . ,-. A semiannual journal of research devoted io He/m/nfho/ogy and aJ/ branches of Parasifo/ogy ''^--, '^ -^ -'/ 'lj,,:':'--' •• r\.L; / .'-•;..•• ' , -N Supported in partly the % BraytonH. Ransom :Memorial Trust Fund r ;':' />•!',"••-•, .' .'.• • V''' ". .r -,'"'/-..•" - V .. ; Subscription $15.00 x« Volume; Foreign, $15J50 ACHOLONU, AtEXANDER D. Hehnihth Fauria of Saurians from Puertox Rico>with \s on the liife Cycle of Lueheifr inscripta (Weslrurrib, 1821 ) and Description of Allopharynx puertoficensis sp. n ....... — — — ,... _.J.-i.__L,.. 106 BERGSTROM, R. C., L. R. tE^AKi AND B. A. WERNER. ^JSmall Dung , Beetles as Biolpgical Control Agents: laboratory Studies of Beetle Action on Tricho- strongylid Eggs in Sheep and Cattle Feces „ ____ ---i.--— .— _..r-..........,_: ______ .... ,171 ^CAKE, EDVWN W., JR. A Key" to Iiarval;Cestodes of Shallow-water, Benthic , ~ . Mollusks of the Northern Gulf 'bf Mexico ... .„'„_ „». -L......^....:,...^;.... _____ ..1.^..... 160 DAVIDSON, WILLIAM R. Endopa'rasjites of Selected Populations of Gray Squir- rels ( Sciurus carolinensis) in the Southeastern United States „;.„.„ ____ i ____ .... 211 DORAN, D. J. AND P: C. AUGUSTINE. / Eimeria tenella: Comparative Oocyst ;> i; Production in Primary Cultures of Chicken Kidney Cells Maintained in •\s Media Systems ^.......^.L...,.....J..^hL.. ____; C.^i,.^^..... ____ ..7._u......;. 126 cEssER,^R. P., V. Q.^PERRY AND A. L. TAYLOR. A '-Diagnostic Compendium of the _ Genus Meloidogyne ([Nematoda: Heteroderidae ) .... .... ... y— ..L_^...-...,_... ___ ...v , 138 EISCHTHAL, JACOB H. AND .ALEXANDER D. AciiOLONy. Some Digenetic Trem- ' atodes from the Atlantic UHawksbill Turtle,' Eretmochdys inibricata ^ /irribrieaia (L.), from Puerto Rico ~L^ _____ ,:,.......„._: ____ , _______ . -

Malacofauna Marina Del Parque Nacional “Los Caimanes”, Villa Clara, Cuba
Tesis de Diploma Malacofauna Marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Junio, 2011 Universidad Central “Marta Abreu” de Las Villas Facultad Ciencias Agropecuaria TESIS DE DIPLOMA Malacofauna marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Tutor: M. C. Ángel Quirós Espinosa Investigador Auxiliar y Profesor Auxiliar [email protected] Centro de Estudios y Servicios Ambientales, CITMA-Villa Clara Carretera Central 716, Santa Clara Consultante: Dr.C. José Espinosa Sáez Investigador Titular Instituto de Oceanología Junio, 2011 Pensamiento “La diferencia entre una mala observación y una buena, es que la primera es errónea y la segunda es incompleta.” Van Hise Dedicatoria Dedicatoria: A mis padres, a Yandy y a mi familia: por las innumerables razones que me dan para vivir, y por ser fuente de inspiración para mis metas. Agradecimientos Agradecimientos: Muchos son los que de alguna forma contribuyeron a la realización de este trabajo, todos saben cuánto les agradezco: Primero quiero agradecer a mis padres, que aunque no estén presentes sé que de una forma u otra siempre estuvieron allí para darme todo su amor y apoyo. A mi familia en general: a mi abuela, hermano, a mis tíos por toda su ayuda y comprensión. A Yandy y a su familia que han estado allí frente a mis dificultades. Agradecer a mi tutor el M.Sc. Ángel Quirós, a mi consultante el Dr.C. José Espinosa y a la Dra.C. María Elena, por su dedicación para el logro de esta tesis. A mis compañeros de grupo por estos cinco años que hemos compartidos juntos, que para mí fueron inolvidables. -

Studies on Molluscan Shells: Contributions from Microscopic and Analytical Methods
Micron 40 (2009) 669–690 Contents lists available at ScienceDirect Micron journal homepage: www.elsevier.com/locate/micron Review Studies on molluscan shells: Contributions from microscopic and analytical methods Silvia Maria de Paula, Marina Silveira * Instituto de Fı´sica, Universidade de Sa˜o Paulo, 05508-090 Sa˜o Paulo, SP, Brazil ARTICLE INFO ABSTRACT Article history: Molluscan shells have always attracted the interest of researchers, from biologists to physicists, from Received 25 April 2007 paleontologists to materials scientists. Much information is available at present, on the elaborate Received in revised form 7 May 2009 architecture of the shell, regarding the various Mollusc classes. The crystallographic characterization of Accepted 10 May 2009 the different shell layers, as well as their physical and chemical properties have been the subject of several investigations. In addition, many researches have addressed the characterization of the biological Keywords: component of the shell and the role it plays in the hard exoskeleton assembly, that is, the Mollusca biomineralization process. All these topics have seen great advances in the last two or three decades, Shell microstructures expanding our knowledge on the shell properties, in terms of structure, functions and composition. This Electron microscopy Infrared spectroscopy involved the use of a range of specialized and modern techniques, integrating microscopic methods with X-ray diffraction biochemistry, molecular biology procedures and spectroscopy. However, the factors governing synthesis Electron diffraction of a specific crystalline carbonate phase in any particular layer of the shell and the interplay between organic and inorganic components during the biomineral assembly are still not widely known. This present survey deals with microstructural aspects of molluscan shells, as disclosed through use of scanning electron microscopy and related analytical methods (microanalysis, X-ray diffraction, electron diffraction and infrared spectroscopy). -

Florida Keys Species List
FKNMS Species List A B C D E F G H I J K L M N O P Q R S T 1 Marine and Terrestrial Species of the Florida Keys 2 Phylum Subphylum Class Subclass Order Suborder Infraorder Superfamily Family Scientific Name Common Name Notes 3 1 Porifera (Sponges) Demospongia Dictyoceratida Spongiidae Euryspongia rosea species from G.P. Schmahl, BNP survey 4 2 Fasciospongia cerebriformis species from G.P. Schmahl, BNP survey 5 3 Hippospongia gossypina Velvet sponge 6 4 Hippospongia lachne Sheepswool sponge 7 5 Oligoceras violacea Tortugas survey, Wheaton list 8 6 Spongia barbara Yellow sponge 9 7 Spongia graminea Glove sponge 10 8 Spongia obscura Grass sponge 11 9 Spongia sterea Wire sponge 12 10 Irciniidae Ircinia campana Vase sponge 13 11 Ircinia felix Stinker sponge 14 12 Ircinia cf. Ramosa species from G.P. Schmahl, BNP survey 15 13 Ircinia strobilina Black-ball sponge 16 14 Smenospongia aurea species from G.P. Schmahl, BNP survey, Tortugas survey, Wheaton list 17 15 Thorecta horridus recorded from Keys by Wiedenmayer 18 16 Dendroceratida Dysideidae Dysidea etheria species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 19 17 Dysidea fragilis species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 20 18 Dysidea janiae species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 21 19 Dysidea variabilis species from G.P. Schmahl, BNP survey 22 20 Verongida Druinellidae Pseudoceratina crassa Branching tube sponge 23 21 Aplysinidae Aplysina archeri species from G.P. Schmahl, BNP survey 24 22 Aplysina cauliformis Row pore rope sponge 25 23 Aplysina fistularis Yellow tube sponge 26 24 Aplysina lacunosa 27 25 Verongula rigida Pitted sponge 28 26 Darwinellidae Aplysilla sulfurea species from G.P. -

(Amblypygi) Provide Insights Into Arachnid Genome Evolution and Antenniform Leg Patterning Guilherme Gainett* and Prashant P
Gainett and Sharma EvoDevo (2020) 11:18 https://doi.org/10.1186/s13227-020-00163-w EvoDevo RESEARCH Open Access Genomic resources and toolkits for developmental study of whip spiders (Amblypygi) provide insights into arachnid genome evolution and antenniform leg patterning Guilherme Gainett* and Prashant P. Sharma Abstract Background: The resurgence of interest in the comparative developmental study of chelicerates has led to important insights, such as the discovery of a genome duplication shared by spiders and scorpions, inferred to have occurred in the most recent common ancestor of Arachnopulmonata (a clade comprising the fve arachnid orders that bear book lungs). Nonetheless, several arachnid groups remain understudied in the context of develop- ment and genomics, such as the order Amblypygi (whip spiders). The phylogenetic position of Amblypygi in Arach- nopulmonata posits them as an interesting group to test the incidence of the proposed genome duplication in the common ancestor of Arachnopulmonata, as well as the degree of retention of duplicates over 450 Myr. Moreover, whip spiders have their frst pair of walking legs elongated and modifed into sensory appendages (a conver- gence with the antennae of mandibulates), but the genetic patterning of these antenniform legs has never been investigated. Results: We established genomic resources and protocols for cultivation of embryos and gene expression assays by in situ hybridization to study the development of the whip spider Phrynus marginemaculatus. Using embryonic tran- scriptomes from three species of Amblypygi, we show that the ancestral whip spider exhibited duplications of all ten Hox genes. We deploy these resources to show that paralogs of the leg gap genes dachshund and homothorax retain arachnopulmonate-specifc expression patterns in P. -

Tactile Learning by a Whip Spider, <I>Phrynus Marginemaculatus</I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Eileen Hebets Publications Papers in the Biological Sciences 4-2009 Tactile learning by a whip spider, Phrynus marginemaculatus C. L. Koch (Arachnida, Amblypygi) Roger D. Santer University of Limerick, Ireland, [email protected] Eileen Hebets University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscihebets Part of the Behavior and Ethology Commons Santer, Roger D. and Hebets, Eileen, "Tactile learning by a whip spider, Phrynus marginemaculatus C. L. Koch (Arachnida, Amblypygi)" (2009). Eileen Hebets Publications. 47. https://digitalcommons.unl.edu/bioscihebets/47 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Eileen Hebets Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 195:4 (April 2009), pp. 393-399; doi: 10.1007/s00359-009-0417-8 Copyright © Springer-Verlag 2009. Used by permission. Submitted October 27, 2008; revised January 12, 2009; accepted January 16, 2009; published online February 7, 2009. Tactile learning by a whip spider, Phrynus marginemaculatus C. L. Koch (Arachnida, Amblypygi) Roger D. Santer1, 2 and Eileen A. Hebets1 1. School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, NE 68588, USA 2. Department of Life Sciences, Schrödinger Building, University of Limerick, Limerick, Ireland Corresponding author — Roger D. Santer, email [email protected] Abstract The ability of animals to learn and remember underpins many behavioral actions and can be crucial for survival in certain contexts, for example in finding and recognizing a habitual refuge. -
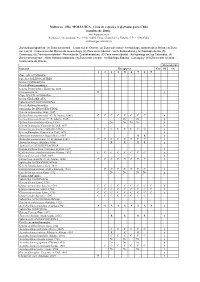
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Flut Und Hitze: Auswirkungen Extremer Klimaereignisse Auf Die
Flut und Hitze: Auswirkungen extremer Klimaereignisse auf die epigäische Arthropodenfauna (Araneae – Spinnen) ufernaher Lebensräume (Auen, Polder) des Inselrheins bei Mainz Dissertation zur Erlangung des Grades „Doktor der Naturwissenschaften“ am Fachbereich Biologie der Johannes-Gutenberg-Universität in Mainz Patrick Guhmann geb. in Ludwigshafen am Rhein Mainz, im Dezember 2009 Tag der mündlichen Prüfung: 11.05.2010 Überall geht ein früheres Ahnen dem späteren Wissen voraus... (Alexander Freiherr von Humboldt) Lebenslauf Persönliche Daten: Vor- und Zuname: Patrick Guhmann Geburtstag: 25.05.1978 Geburtsort: Ludwigshafen am Rhein Wohnort: Sachsenstr. 5a, 67134 Birkenheide Staatsangehörigkeit: deutsch Familienstand: ledig Schulbildung: 1984 - 1988 Albertine-Scherer-Schule Birkenheide 1988 - 1996 Carl-Bosch-Gymnasium Ludwigshafen 1996 - 1999 Max-Planck-Gymnasium Ludwigshafen Wehrdienst: 1. Juli 1999 - 30. Sept. 2000 Bundesmarine Studium: Studienbeginn am 01.10.2000 im Studienfach Biologie an der Johannes-Gutenberg Universität Mainz Erfolgreicher Abschluss des Grundstudiums mit dem Vordiplom am 19.11.2002 an der Johannes-Gutenberg Universität Mainz Erfolgreicher Studienabschluss an der Johannes-Gutenberg Universität Mainz mit dem Diplom am 09.12.2005 Beginn der Promotion am 18.09.2006 an der Johannes-Gutenberg Universität Mainz Hiermit erkläre ich, die vorliegende Arbeit selbständig und nur unter Verwendung der angegebenen Quellen und Hilfsmittel angefertigt zu haben. Patrick Guhmann, Mainz, im Dezember 2009 Inhaltsverzeichnis 1. Einleitung -

Back Matter (PDF)
Index of subjects Page numbers in italics refer to figures; those in bold type to tables. abdominal sense organ 49, 50 bio-indicators, mussels as 469-476 abfrontal surface 273-278 biodiversity, mussels as indicators 469, 470 acrosomal complex 170 biofouling 474 acrosomes 176, 177, 181 biogeography, Mytilus galloprovincialis 389-397 Pteriomorphia 182 bird predation, on Ensis directus 459-464 tridacnids 193, 197, 198 bivalve size, seep communities 238-239 actinodonty 61 bootstrap test 117 Adams consensus tree, Palaeozoic taxa 55 tree, rudists 119 adductor muscle 47, 59 branch swapping 24, 35 Lucinidae 210, 217-218 Bray-Curtis analysis 380, 381,382,384 adoral sense organ 50 brooding 171, 184 Aequipecten opercularis, and palaeoenvironments 425-439 Buckhorn Asphalt 291-301 allele frequencies, Mytilus galloprovincialis 389, 390, map 292 391,393, 394 burrowing depth allometrics 403,406-407,421 and flesh growth 452 allozyme studies 153, 389 Macoma balthica 451-458, 453, 454, 455 ammonites 234 burrowing habit anaerobic capability 209 anomalodesmatans 129 Anomalodesmata 12, 21, 25, 53 chemosymbionts 209, 222 carnivory 132, 133 byssus characters 133-134, 138, 139 Lyonsiidae 129 cladistic studies 129-143 retention of 89, 466 data matrix 140 scallops 251 evolution 64 Teranota 341 families 129, 130 fossil record 134-136, 135 calcium granules 329-337,331,333, 334 orders 34 calcium phosphate 329, 335-336 phylogeny 339-346 carbon isotopes 430--431 relations 44 carbonate muds 353 species lists 130 carnivory strict consensus tree 137 anomalodesmatans 132 -

East Coast Marine Shells; Descriptions of Shore Mollusks Together With
fi*": \ EAST COAST MARINE SHELLS / A • •:? e p "I have seen A curious child, who dwelt upon a tract Of Inland ground, applying to his ear The .convolutions of a smooth-lipp'd shell; To yi'hJ|3h in silence hush'd, his very soul ListehM' .Intensely and his countenance soon Brightened' with joy: for murmerings from within Were heai>^, — sonorous cadences, whereby. To his b^ief, the monitor express 'd Myster.4?>us union with its native sea." Wordsworth 11 S 6^^ r EAST COAST MARINE SHELLS Descriptions of shore mollusks together with many living below tide mark, from Maine to Texas inclusive, especially Florida With more than one thousand drawings and photographs By MAXWELL SMITH EDWARDS BROTHERS, INC. ANN ARBOR, MICHIGAN J 1937 Copyright 1937 MAXWELL SMITH PUNTZO IN D,S.A. LUhoprinted by Edwards B'olheri. Inc.. LUhtiprinters and Publishert Ann Arbor, Michigan. iQfj INTRODUCTION lilTno has not felt the urge to explore the quiet lagoon, the sandy beach, the coral reef, the Isolated sandbar, the wide muddy tidal flat, or the rock-bound coast? How many rich harvests of specimens do these yield the collector from time to time? This volume is intended to answer at least some of these questions. From the viewpoint of the biologist, artist, engineer, or craftsman, shellfish present lessons in development, construction, symme- try, harmony and color which are almost unique. To the novice an acquaint- ance with these creatures will reveal an entirely new world which, in addi- tion to affording real pleasure, will supply much of practical value. Life is indeed limitless and among the lesser animals this is particularly true.