Vulnerability Assessment of the North East Atlantic Shelf Marine Ecoregion to Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Critically Endangered - Wikipedia
Critically endangered - Wikipedia Not logged in Talk Contributions Create account Log in Article Talk Read Edit View history Critically endangered From Wikipedia, the free encyclopedia Main page Contents This article is about the conservation designation itself. For lists of critically endangered species, see Lists of IUCN Red List Critically Endangered Featured content species. Current events A critically endangered (CR) species is one which has been categorized by the International Union for Random article Conservation status Conservation of Nature (IUCN) as facing an extremely high risk of extinction in the wild.[1] Donate to Wikipedia by IUCN Red List category Wikipedia store As of 2014, there are 2464 animal and 2104 plant species with this assessment, compared with 1998 levels of 854 and 909, respectively.[2] Interaction Help As the IUCN Red List does not consider a species extinct until extensive, targeted surveys have been About Wikipedia conducted, species which are possibly extinct are still listed as critically endangered. IUCN maintains a list[3] Community portal of "possibly extinct" CR(PE) and "possibly extinct in the wild" CR(PEW) species, modelled on categories used Recent changes by BirdLife International to categorize these taxa. Contact page Contents Tools Extinct 1 International Union for Conservation of Nature definition What links here Extinct (EX) (list) 2 See also Related changes Extinct in the Wild (EW) (list) 3 Notes Upload file Threatened Special pages 4 References Critically Endangered (CR) (list) Permanent -
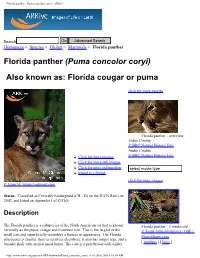
Florida Panther - Puma Concolor Coryi - Arkive
Florida panther - Puma concolor coryi - ARKive Search Homepage > Species > Global > Mammals > Florida panther Florida panther (Puma concolor coryi) Also known as: Florida cougar or puma click for more movies Florida panther - overview Video Credits: © BBC Natural History Unit Audio Credits: © BBC Natural History Unit ● Click for more movies ● Click for more still images ● Click for more information ● Email to a friend click for more images © Lynn M. Stone / naturepl.com Status: Classified as Critically Endangered (CR - D) on the IUCN Red List 2002, and listed on Appendix I of CITES. Description The Florida panther is a subspecies of the North American cat that is known Florida panther - 3 weeks old variously as the puma, cougar and mountain lion. This is the largest of the © Frank Schneidermeyer / OSF / small cats and superficially resembles a lioness in appearance. The Florida Photolibrary.com subspecies is smaller than its relatives elsewhere; it also has longer legs, and a [ medium ] [ large ] broader skull with arched nasal bones. The coat is a pale brown with whiter http://www.arkive.org/species/GES/mammals/Puma_concolor_coryi/ (1 of 2)4/6/2005 8:16:04 AM Florida panther - Puma concolor coryi - ARKive underparts and a black tip at the end of the long tail. Infants have a spotted coat and blue eyes. Florida panthers often have crooked ends to their tails, and whorls of hair on their backs; these are thought not to be characteristic of the subspecies however, and may be signs of inbreeding. Click for more information Florida panther - 5 months old © Bob Bennett / OSF / Photolibrary.com [ medium ] [ large ] © Wildscreen 2004 By using this website you agree to the Terms of Use About ARKive | Competition | Contact | Newsletter | FAQ | Links http://www.arkive.org/species/GES/mammals/Puma_concolor_coryi/ (2 of 2)4/6/2005 8:16:04 AM. -
Endangered Species
Not logged in Talk Contributions Create account Log in Article Talk Read Edit View history Endangered species From Wikipedia, the free encyclopedia Main page Contents For other uses, see Endangered species (disambiguation). Featured content "Endangered" redirects here. For other uses, see Endangered (disambiguation). Current events An endangered species is a species which has been categorized as likely to become Random article Conservation status extinct . Endangered (EN), as categorized by the International Union for Conservation of Donate to Wikipedia by IUCN Red List category Wikipedia store Nature (IUCN) Red List, is the second most severe conservation status for wild populations in the IUCN's schema after Critically Endangered (CR). Interaction In 2012, the IUCN Red List featured 3079 animal and 2655 plant species as endangered (EN) Help worldwide.[1] The figures for 1998 were, respectively, 1102 and 1197. About Wikipedia Community portal Many nations have laws that protect conservation-reliant species: for example, forbidding Recent changes hunting , restricting land development or creating preserves. Population numbers, trends and Contact page species' conservation status can be found in the lists of organisms by population. Tools Extinct Contents [hide] What links here Extinct (EX) (list) 1 Conservation status Related changes Extinct in the Wild (EW) (list) 2 IUCN Red List Upload file [7] Threatened Special pages 2.1 Criteria for 'Endangered (EN)' Critically Endangered (CR) (list) Permanent link 3 Endangered species in the United -

EARTH Ltd PME Threatened Habitats Handout
501-C-3 at Southwick’s Zoo, 2 Southwick Street, Mendon, MA 01756 PROTECTING MY EARTH: LOCALLY THREATENED HABITATS (MA) FACTS & FIGURES “Protecting My EARTH” is an environmental education program offered by EARTH Ltd. to help students learn how to take better care of their community and their planet. KEY TERMS AND DEFINITIONS • Conservation: a careful preservation and protection of something; especially planned management of a natural resource to prevent exploitation, destruction, or neglect • Habitat: the place or environment where a plant or animal naturally or normally lives and grows • Ecosystem: everything that exists in a particular environment • Endangered: a species in danger of becoming extinct • Extinct: no longer existing • Threatened: having an uncertain chance of continued survival; likely to become an endangered species • Vulnerable: easily damaged; likely to become an endangered species • CITES - Convention on International Trade of Endangered Species of Wild Fauna and Flora: an international agreement between governments effective since 1975. Its aim is to ensure that international trade in specimens of wild animals and plants does not threaten their survival. Roughly 5,600 species of animals and 30,000 species of plants are protected by CITES as of 2013. There are currently 181 countries (of about 196) that are contracting parties. • IUCN - International Union for the Conservation of Nature: world’s oldest and largest global environmental organization, with almost 1,300 government and NGO Members and more than 15,000 volunteer experts in 185 countries. Their work focuses on valuing and conserving nature, ensuring effective and equitable governance of its use, and deploying nature-based solutions to global challenges in climate, food and development. -

Fueling Extinction: How Dirty Energy Drives Wildlife to the Brink
Fueling Extinction: How Dirty Energy Drives Wildlife to the Brink The Top Ten U.S. Species Threatened by Fossil Fuels Introduction s Americans, we are living off of energy sources produced That hasn’t stopped oil and gas companies from gobbling in the age of the dinosaurs. Fossil fuels are dirty. They’re up permits and leases for millions of acres of our pristine Adangerous. And, they’ve taken an incredible toll on our public land, which provides important wildlife habitat and country in many ways. supplies safe drinking water to millions of Americans. And the industry is demanding ever more leases, even though it is Our nation’s threatened and endangered wildlife, plants, birds sitting on thousands of leases it isn’t using—an area the size of and fish are among those that suffer from the impacts of our Pennsylvania. fossil fuel addiction in the United States. This report highlights ten species that are particularly vulnerable to the pursuit Oil companies have generated billions of dollars in profits, and of oil, gas and coal. Our outsized reliance on fossil fuels and paid their senior executives $220 million in 2010 alone. Yet the impacts that result from its development, storage and ExxonMobil, Chevron, Shell, and BP combined have reduced transportation is making it ever more difficult to keep our vow to their U.S. workforce by 11,200 employees since 2005. protect America’s wildlife. The American people are clearly getting the short end of the For example, the Arctic Ocean is home to some of our most stick from the fossil fuel industry, both in terms of jobs and in beloved wildlife—polar bears, whales, and seals. -

26Th International Congress for Conservation Biology
Program SocietySociety for for Conservation Conservation Biology Biology 26th International Congress for Conservation Biology Connecting Systems, Disciplines, and Stakeholders Baltimore, Maryland, USA • July 21-25, 2013 www.conbio.org/2013 Baltimore,2013 Maryland, USA Auckland,2011 New Zealand Edmonton,2010 Alberta, Canada Beijing,2009 China Chattanooga,2008 Tennessee, USA Port2007 Elizabeth, South Africa About the International Congress for Conservation Biology San2006 Jose, California, USA Welcome to our international forum for addressing conservation challenges. The International Congress for Conservation Biology Universidade2005 de Brasília, Brasília, Brazil is the global gathering spot for presenting and discussing new research and developments in conservation science and practice. From North America to Asia and Oceania to Europe, ICCB Columbia2004 University, New York, New York, USA moves around the world and is recognized as the most important global meeting for conservation professionals and students. University2003 of Minnesota, Duluth, Minnesota, USA Most importantly, the ICCB connects conservation professionals and serves as the premier networking opportunity for anyone University2002 of Kent at Canterbury, United Kingdom interested in conservation. University2001 of Hawaii, Hilo, Hawaii, USA About the Society for Conservation Biology Dedicated to advancing the science and practice of conserving University2000 of Montana, Missoula, Montana, USA Earth’s biological diversity, SCB is a global community of conservation professionals -

Species Biodiversity
SPECIES: THE CORNERSTONE OFBIODIVERSITY AN EXAMINATION OF HOW SPECIES DIVERSITY IS THE KEY TO A HEALTHY PLANET, AND A CLOSER LOOK AT A MAJOR TOOL USED IN BIODIVERSITY CONSERVATION 4 Kathryn Pintus, IUCN So far, we’ve had a look at genetic diversity, and we’ve learned that genes are responsible for the wide variety of species that exist on Earth. But what exactly is a species? A species is a basic biological unit, describing organisms which are able to breed together and produce fertile offspring (offspring that are able to produce young). The above statement is a fairly widely accepted definition, and in some cases it is easy enough to determine whether two organisms are separate species simply by looking at them; the mighty blue whale is clearly not the same species as the fly agaric mushroom. A BANANA SLUG EATING A RASPBERRY AT A CAMPSITE IN REDWOOD NATIONAL PaRK, CALIFORNIA, USA. © Anthony Avellano (age 14) 39 CHAPTER 4 | Species: the cornerstone of biodiversity However, the situation is not taxonomy, and this provides and where it lives), and others always quite so straightforward. us with a common language that still on phylogenetics (using The science of describing and we can all use to communicate molecular genetics to look at classifying organisms is called about species, but it can get evolutionary relatedness). For rather complicated! Biology is this reason, when considering split into several fields, including two organisms which on botany, zoology, ecology, the surface may look almost genetics and behavioural identical, scientists sometimes science, and scientists from each disagree as to how to classify of these branches of biology will them. -

WILDLIFE in a CHANGING WORLD an Analysis of the 2008 IUCN Red List of Threatened Species™
WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart coberta.indd 1 07/07/2009 9:02:47 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ first_pages.indd I 13/07/2009 11:27:01 first_pages.indd II 13/07/2009 11:27:07 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart first_pages.indd III 13/07/2009 11:27:07 The designation of geographical entities in this book, and the presentation of the material, do not imply the expressions of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily refl ect those of IUCN. This publication has been made possible in part by funding from the French Ministry of Foreign and European Affairs. Published by: IUCN, Gland, Switzerland Red List logo: © 2008 Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Vié, J.-C., Hilton-Taylor, C. -

Wildscreen and Arkive Project
Wildscreen and ARKive Project ARKive is the world’s centralised library of films and photographs of all the U.K. and worldwide endangered species – freely accessible to all online. Hailed as the digital Noah’s Ark, it has won numerous education and communication awards (and was launched by Sir David Attenborough). Here are details of the ARKive website: • The main ARKive website www.arkive.org is designed for use by 11 year olds upwards. Its content consists of the largest collection of wildlife and environmental films and images that can be viewed for free. The content is relevant to a wide range of science and geography subjects in primary and secondary schools (all can be downloaded free of charge). • Planet ARKive www.planetarkive.org is for children aged 7-11, and designed to make life science learning and environmental education a widely enjoyable experience. It fits in especially well with learning about living things in their environment for KS2 and 3. Again it is free and fun to use • ARKive Education www.arkiveeducation.org is for teachers– and offers downloadable briefings, lesson plans and project ideas to support KS 2 and 3 National Curriculum leaning targets. It too is free to use. Any feedback would be much appreciated - especially on how I can help young people to use the sites more effectively, and the types of resources that they would like added to the sites. If you know of anyone who would be interested in working more closely with ARKive, contact me: [email protected] Please do get in contact for further information or if I can assist you in any way. -

Vital but Vulnerable: Climate Change Vulnerability and Human Use of Wildlife in Africa’S Albertine Rift
Vital but vulnerable: Climate change vulnerability and human use of wildlife in Africa’s Albertine Rift J.A. Carr, W.E. Outhwaite, G.L. Goodman, T.E.E. Oldfield and W.B. Foden Occasional Paper for the IUCN Species Survival Commission No. 48 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN or the compilers concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2013 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Carr, J.A., Outhwaite, W.E., Goodman, G.L., Oldfield, T.E.E. and Foden, W.B. 2013. Vital but vulnerable: Climate change vulnerability and human use of wildlife in Africa’s Albertine Rift. Occasional Paper of the IUCN Species Survival Commission No. 48. IUCN, Gland, Switzerland and Cambridge, UK. xii + 224pp. ISBN: 978-2-8317-1591-9 Front cover: A Burundian fisherman makes a good catch. © R. Allgayer and A. Sapoli. Back cover: © T. Knowles Available from: IUCN (International Union for Conservation of Nature) Publications Services Rue Mauverney 28 1196 Gland Switzerland Tel +41 22 999 0000 Fax +41 22 999 0020 [email protected] www.iucn.org/publications Also available at http://www.iucn.org/dbtw-wpd/edocs/SSC-OP-048.pdf About IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. -

Don E. Wilson, Russell A. Mittermeier (Eds.): Handbook of the Mammals Of
jsou natolik výrazné, že mohou opravdu Jan Robovský a kolektiv RECENZE překvapit, byť sám způsob změn už nikoli (fylogenetický koncept je všeobecně znám, viz také Vesmír 2007, 9: 568 –571). Důvody k povýšení některých popula - Don E. Wilson, Russell A. Mittermeier (Eds.): cí/poddruhů na druhovou úroveň jsou zmíněny v knize Ungulate Taxonomy od Handbook of the Mammals of the World Colina P. Grovese a Petera Grubba, vydané rovněž v r. 2011. Její výhodou je, že kopyt - – Volume 2, Hoofed Mammals (Kopytníci) níky revidovala jednotně s pomocí fyloge - netického konceptu, a proto ji považujeme z hlediska taxonomie za užitečnější a smě - Nejen srdce všech zoologů jistě potěšil podobně zapůsobí nejvíce, je její výrazná rodatnější než recenzovanou encyklope - druhý svazek osmidílné encyklopedie nevyrovnanost v užité taxonomii. Zatímco dii. V nyní hodnocené knize došlo navíc o savcích světa věnovaný kopytníkům. Je většina skupin je zpracována v různé míře k řadě změn na úrovni rodů, ale opět ne - podstatně rozsáhlejší než první díl o šel - ještě s využitím tzv. biologického koncep - konzistentně (např. tapír čabrakový zde mách (recenzoval J. Suchomel v Živě 2009, tu druhu, u turovitých ( Bovidae ), kančilo - není Acrocodia indica , zatímco kudu malý 6: XCVII –XCVIII); ten byl překonán téměř vitých ( Tragulidae ), kabarovitých ( Mos - má vlastní rod Ammelaphus apod.). Tyto o 160 stran. Jde bezesporu o významné chidae ) a slonovitých ( Elephantidae ) je rozdíly jsou velice nápadné a je třeba při - dílo poskytující množství užitečných in - naplno využit nový a progresivní fyloge - znat, že nejednotná koncepce druhů před - formací doprovázených jak kvalitními netický koncept (pro vysvětlení rozdílů stavuje velké editorské selhání. Malou útě - fotografiemi, tak vydařenými obrazovými viz doplňující podkapitola v závěru recen - chou může být fakt, že jsou u ostatních tabulemi. -

LESSONS in CONSERVATION VOLUME 9 STUDIO JANUARY 2019 ISSUE Network of Conservation Educators & Practitioners
ISSN: 1938-7024 Center for Biodiversity and Conservation Network of Conservation Educators & Practitioners LESSONS IN CONSERVATION VOLUME 9 STUDIO JANUARY 2019 ISSUE Network of Conservation Educators & Practitioners Lessons in Conservation is the official journal of the Network of Conservation Educators and Practitioners (NCEP)—a collaborative project of the Center for Biodiversity and Conservation (CBC) at the American Museum of Natural History—and is published as issues become available. Teaching and learning modules presented here in Lessons in Conservation are available in modifiable form for teachers on the NCEP website (ncep.amnh.org). All materials are distributed free of charge. Any opinions, findings, and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the American Museum of Natural History or the funders of this project. All components of a module (Syntheses, Exercises, and Case Studies) have been peer-reviewed and approved for publication by NCEP. Editors: Production team: Kristin Douglas Ana L. Porzecanski NCEP Production and Outreach Coordinator CBC and NCEP Director Suzanne Macey Nadav Gazit NCEP Manager CBC and NCEP Visual Creative and Research Assistant Kimberley Landrigan CBC Capacity Development Specialist Stefanie Siller NCEP Intern Lessons in Conservation is available online at: ncep.amnh.org/linc All reproduction or distribution must provide full citation of the original work and provide a copyright notice as follows: “Copyright 2019,