Techniques to Estimate Radiation Dose to Skin During Fluoroscopically Guided Procedures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Personal Radiation Monitoring
Personal Radiation Monitoring Tim Finney 2020 Radiation monitoring Curtin staff and students who work with x-ray machines, neutron generators, or radioactive substances are monitored for exposure to ionising radiation. The objective of radiation monitoring is to ensure that existing safety procedures keep radiation exposure As Low As Reasonably Achievable (ALARA). Personal radiation monitoring badges Radiation exposure is measured using personal radiation monitoring badges. Badges contain a substance that registers how much radiation has been received. Here is the process by which a user’s radiation dose is measured: 1. The user is given a badge to wear 2. The user wears the badge for a set time period (usually three months) 3. At the end of the set time, the user returns the badge 4. The badge is sent away to be read 5. A dose report is issued. These steps are repeated until monitoring is no longer required. Badges are supplied by a personal radiation monitoring service provider. Curtin uses a service provider named Landauer. In addition to user badges, the service provider sends control badges that are kept on site in a safe place away from radiation sources. The service provider reads each badge using a process that extracts a signal from the substance contained in the badge to obtain a dose measurement. (Optically stimulated luminescence is one such process.) The dose received by the control badge is subtracted from the user badge reading to obtain the user dose during the monitoring period. Version 1.0 Uncontrolled document when printed Health and Safety Page 1 of 7 A personal radiation monitoring badge Important Radiation monitoring badges do not protect you from radiation exposure. -

Proper Use of Radiation Dose Metric Tracking for Patients Undergoing Medical Imaging Exams
Proper Use of Radiation Dose Metric Tracking for Patients Undergoing Medical Imaging Exams Frequently Asked Questions Introduction In August of 2021, the American Association of Physicists in Medicine (AAPM), the American College of Radiology (ACR), and the Health Physics Society (HPS) jointly released the following position statement advising against using information about a patient’s previous cumulative dose information from medical imaging exams to decide the appropriateness of future imaging exams. This statement was also endorsed by the Radiological Society of North America (RSNA). It is the position of the American Association of Physicists in Medicine (AAPM), the American College of Radiology (ACR), and the Health Physics Society (HPS) that the decision to perform a medical imaging exam should be based on clinical grounds, including the information available from prior imaging results, and not on the dose from prior imaging-related radiation exposures. AAPM has long advised, as recommended by the International Commission on Radiological Protection (ICRP), that justification of potential patient benefit and subsequent optimization of medical imaging exposures are the most appropriate actions to take to protect patients from unnecessary medical exposures. This is consistent with the foundational principles of radiation protection in medicine, namely that patient radiation dose limits are inappropriate for medical imaging exposures. Therefore, the AAPM recommends against using dose values, including effective dose, from a patient’s prior imaging exams for the purposes of medical decision making. Using quantities such as cumulative effective dose may, unintentionally or by institutional or regulatory policy, negatively impact medical decisions and patient care. This position statement applies to the use of metrics to longitudinally track a patient’s dose from medical radiation exposures and infer potential stochastic risk from them. -

Cumulative Radiation Dose in Patients Admitted with Subarachnoid Hemorrhage: a Prospective PATIENT SAFETY Study Using a Self-Developing Film Badge
Cumulative Radiation Dose in Patients Admitted with Subarachnoid Hemorrhage: A Prospective PATIENT SAFETY Study Using a Self-Developing Film Badge A.C. Mamourian BACKGROUND AND PURPOSE: While considerable attention has been directed to reducing the x-ray H. Young dose of individual imaging studies, there is little information available on the cumulative dose during imaging-intensive hospitalizations. We used a radiation-sensitive badge on 12 patients admitted with M.F. Stiefel SAH to determine if this approach was feasible and to measure the extent of their x-ray exposure. MATERIALS AND METHODS: After obtaining informed consent, we assigned a badge to each of 12 patients and used it for all brain imaging studies during their ICU stay. Cumulative dose was deter- mined by quantifying exposure on the badge and correlating it with the number and type of examinations. RESULTS: The average skin dose for the 3 patients who had only diagnostic DSA without endovascular intervention was 0.4 Gy (0.2–0.6 Gy). The average skin dose of the 8 patients who had both diagnostic DSA and interventions (eg, intra-arterial treatment of vasospasm and coiling of aneurysms) was 0.9 Gy (1.8–0.4 Gy). One patient had only CT examinations. There was no effort made to include or exclude the badge in the working view during interventions. CONCLUSIONS: It is feasible to incorporate a film badge that uses a visual scale to monitor the x-ray dose into the care of hospitalized patients. Cumulative skin doses in excess of 1 Gy were not uncommon (3/12) in this group of patients with acute SAH. -

Radiation Glossary
Radiation Glossary Activity The rate of disintegration (transformation) or decay of radioactive material. The units of activity are Curie (Ci) and the Becquerel (Bq). Agreement State Any state with which the U.S. Nuclear Regulatory Commission has entered into an effective agreement under subsection 274b. of the Atomic Energy Act of 1954, as amended. Under the agreement, the state regulates the use of by-product, source, and small quantities of special nuclear material within said state. Airborne Radioactive Material Radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases. ALARA Acronym for "As Low As Reasonably Achievable". Making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken. It takes into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, societal and socioeconomic considerations, and in relation to utilization of radioactive materials and licensed materials in the public interest. Alpha Particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, with a mass number of 4 and a charge of +2. Annual Limit on Intake (ALI) Annual intake of a given radionuclide by "Reference Man" which would result in either a committed effective dose equivalent of 5 rems or a committed dose equivalent of 50 rems to an organ or tissue. Attenuation The process by which radiation is reduced in intensity when passing through some material. -

``Low Dose``And/Or``High Dose``In Radiation Protection: a Need To
IAEA-CN-67/154 "LOW DOSE" AND/OR "HIGH DOSE" IN RADIATION PROTECTION: A NEED TO SETTING CRITERIA FOR DOSE CLASSIFICATION Mehdi Sohrabi National Radiation Protection Department & XA9745670 Center for Research on Elevated Natural Radiation Atomic Energy Organization of Iran, Tehran ABSTRACT The "low dose" and/or "high dose" of ionizing radiation are common terms widely used in radiation applications, radiation protection and radiobiology, and natural radiation environment. Reading the title, the papers of this interesting and highly important conference and the related literature, one can simply raise the question; "What are the levels and/or criteria for defining a low dose or a high dose of ionizing radiation?". This is due to the fact that the criteria for these terms and for dose levels between these two extreme quantities have not yet been set, so that the terms relatively lower doses or higher doses are usually applied. Therefore, setting criteria for classification of radiation doses in the above mentioned areas seems a vital need. The author while realizing the existing problems to achieve this important task, has made efforts in this paper to justify this need and has proposed some criteria, in particular for the classification of natural radiation areas, based on a system of dose limitation. In radiation applications, a "low dose" is commonly associated to applications delivering a dose such as given to a patient in a medical diagnosis and a "high dose" is referred to a dose in applications such as radiotherapy, mutation breeding, control of sprouting, control of insects, delay ripening, and sterilization having a range of doses from 1 to 105 Gy, for which the term "high dose dosimetry" is applied [1]. -

Internal and External Exposure Exposure Routes 2.1
Exposure Routes Internal and External Exposure Exposure Routes 2.1 External exposure Internal exposure Body surface From outer space contamination and the sun Inhalation Suspended matters Food and drink consumption From a radiation Lungs generator Radio‐ pharmaceuticals Wound Buildings Ground Radiation coming from outside the body Radiation emitted within the body Radioactive The body is equally exposed to radiation in both cases. materials "Radiation exposure" refers to the situation where the body is in the presence of radiation. There are two types of radiation exposure, "internal exposure" and "external exposure." External exposure means to receive radiation that comes from radioactive materials existing on the ground, suspended in the air, or attached to clothes or the surface of the body (p.25 of Vol. 1, "External Exposure and Skin"). Conversely, internal exposure is caused (i) when a person has a meal and takes in radioactive materials in the food or drink (ingestion); (ii) when a person breathes in radioactive materials in the air (inhalation); (iii) when radioactive materials are absorbed through the skin (percutaneous absorption); (iv) when radioactive materials enter the body from a wound (wound contamination); and (v) when radiopharmaceuticals containing radioactive materials are administered for the purpose of medical treatment. Once radioactive materials enter the body, the body will continue to be exposed to radiation until the radioactive materials are excreted in the urine or feces (biological half-life) or as the radioactivity weakens over time (p.26 of Vol. 1, "Internal Exposure"). The difference between internal exposure and external exposure lies in whether the source that emits radiation is inside or outside the body. -

Radiation Safety
Ionizing Radiation Exposure from Radiologic Imaging Background The current and common unit of measurement of “absorbed” ionizing radiation is a milliSievert (mSv). Chest, abdomen or The increased use of diagnostic imaging requiring the use of pelvis CTs will typically expose one to between 6 – 8 mSv. “ionizing radiation,” the rapidly expanding use of computed tomography in the emergency setting, the introduction Specifically, a CT coronary angiogram may carry an of multi-detector CT units and newly reported concerns overall effective radiation dose in a 20 mSv range; the related to the human consequences of low-level radiation organ equivalent dose is quite different.7 Anthromorphic exposure have revitalized a long-standing concern over the mathematical phantoms have shown that such studies quantification and management of an individual’ s cumulative will expose 20-year-old female breast tissue to an organ 1,2,3 “medical” radiation exposure. Studies have shown that equivalent dose of 75 – 80 mSv, and lung tissue as high as 90 many physicians, including radiologists, have developed a mSv. The cancer incidence for a 20-year-old in this example is misconception that the shorter imaging acquisition times believed to be in the one in 143 range…quite significant! With have resulted in lower doses of radiation, when in fact many improvements in technology and CCTA imaging protocols, times the opposite is true. The multi-detector CT units today, effective dose has been reduced significantly, to less than 10 even with shorter scan times, expose patients to higher doses mSv in many cases. of radiation per scan than earlier units. -

Cumulative Radiation Exposure and Your Patient
Imaging Guideline JANUAry 2013 Cumulative Radiation Exposure and Your Patient This document, developed by Intermountain Healthcare’s Cardiovascular Clinical Program and Imaging Clinical Service, provides information on the cumulative radiation exposure reported in HELP2: the limitations of this information, why Intermountain is measuring and reporting it, tips on interpreting this information, and factors to consider when choosing an imaging procedure. Information in this document (click each item below to skip to that section): Please note that while this 1 What’s included — and not included — in your patient’s document provides evidence- cumulative radiation exposure as reported in HELP2 based information to consider in making treatment 2 Why Intermountain is measuring and reporting cumulative decisions for most patients, radiation exposure your approach should be adapted to meet the needs 3 The risks of radiation exposure of individual patients and 4 Factors to consider when choosing an imaging test situations, and should not 5 Discussing this information with your patient replace clinical judgment. 6 Estimated radiation exposures and lifetime risks from common procedures — a quick reference with resources A brief overview of this 1 WHAT’S INCLUDED IN MY PATIENT’S REPORTED topic is also available. For basic, concise information CUMULATIVE RADIATION EXPOSURE? on radiation exposure and The number reported for each patient: risk, see the brief Physician’s Guide to Radiation Exposure. • Includes four types of relatively higher-dose procedures: CT studies, angiography, nuclear cardiology, and cardiac catheterization procedures. • Begins in mid-2012: Earlier exposures are not included. • Does NOT include: Procedures performed at non-Intermountain facilities, other procedures besides the four listed above, or radiation (oncology) treatments. -
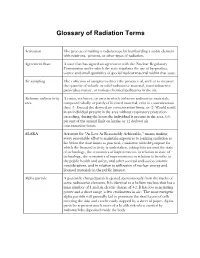
Glossary of Radiation Terms
Glossary of Radiation Terms Activation The process of making a radioisotope by bombarding a stable element with neutrons, protons, or other types of radiation. Agreement State A state that has signed an agreement with the Nuclear Regulatory Commission under which the state regulates the use of by-product, source and small quantities of special nuclear material within that state. Air sampling The collection of samples to detect the presence of, and/or to measure the quantity of volatile or solid radioactive material, non-radioactive particulate matter, or various chemical pollutants in the air. Airborne radioactivity A room, enclosure, or area in which airborne radioactive materials, area composed wholly or partly of licensed material, exist in concentrations that: (1) Exceed the derived air concentration limits, or (2) Would result in an individual present in the area without respiratory protection exceeding, during the hours the individual is present in the area, 0.6 percent of the annual limit on intake or 12 derived air concentration-hours. ALARA Acronym for "As Low As Reasonably Achievable," means making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken, taking into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, and other societal and socioeconomic considerations, and in relation to utilization of nuclear energy and licensed materials in the public interest. Alpha particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. -

Generic Ltrhead-Clinic
Your Annual Dosimetry Badge Summary Report What is this report? This report summarizes the total occupational radiation dose you received from your work with X-rays and radioactive materials at Cornell in the most recent calendar year. Why am I receiving it? State regulations require us to provide individuals monitored for occupational radiation dose an annual summary report of their dose. You can also check your radiation dose online at any time during the year following the instructions at the web portal. What should I do with it? Read the report and contact the Radiation Safety Group if you have questions or concerns: • [email protected] • 607-255-8200 What information is important for me to look at? Your occupational radiation dose from external sources of radiation is summarized on the right side of the form under “Doses (in rem).” The most important values for you to look at are: • Deep Dose Equivalent (DDE): external radiation dose at a tissue depth of 1 cm to the whole body • Lens (Eye) Dose Equivalent (LDE): external radiation dose to the lens of the eye at a tissue depth of 0.3 cm • Shallow Dose Equivalent (SDE,WB): radiation dose to the skin of the whole body at a tissue depth of 0.007 cm • Shallow Dose Equivalent (SDE,ME): radiation dose to the skin of an extremity at a tissue depth of 0.007 cm (arm below the elbow, leg below the knee) • Committed Dose Equivalent (CDE): dose to a specific organ or tissue that will be received from an intake of radioactive material by an individual during the 50-year period following the intake • Committed Effective Dose Equivalent (CEDE): sum of the products of committed dose equivalents for the body organs or tissues multiplied their specific weighting factors What do my numbers mean? State and federal occupational radiation dose limits are set at levels below which there are believed to be negligible health risks to workers. -

Reports Overview
Reports Overview dosimetry services A Mirion Technologies Division Featuring: TABLE OF CONTENTS Overview.................................................................. 1 Report Definition..................................................... 2-3 Occupational Radiation Exposure........................ 4-5 ALARA Exposure.................................................... 6-7 ALARA Letter........................................................... 8 History Detail........................................................... 9 Annual Individual Summary (Form 5)................... 10-11 Statistical Summary................................................ 12 Total Dose............................................................... 13 Dose Management.................................................. 14-17 Preliminary Dose.................................................... 18 Dose Adjustment Confirmation............................. 19 Dose Estimate......................................................... 20-21 Annual Account Summary..................................... 22-23 Unreturned Badge.................................................. 24 Environmental Monitoring Letter.......................... 25 Environmental Monitoring..................................... 26 Report Requests..................................................... 27 OVERVIEW This Reports Overview booklet provides descriptions and samples of the reports that are available through your service with Mirion Technologies Dosimetry Services Division. Mirion Technologies Dosimetry -

Radiation Dose in Radiotherapy from Prescription Deliveryto
IAEA-TECDOC-896 XA9642841 Radiation dose in radiotherapy from prescription deliveryto INTERNATIONAL ATOMIC ENERGY AGENCY The originating Sectio f thino s publicatio IAEe th Ann i was : Dosimetry Section International Atomic Energy Agency Wagramerstrasse5 0 10 x P.OBo . A-1400 Vienna, Austria RADIATION DOSE IN RADIOTHERAPY FROM PRESCRIPTION TO DELIVERY IAEA, VIENNA, 1996 IAEA-TECDOC-896 ISSN 1011-4289 IAEA© , 1996 Printed by the IAEA in Austria August 1996 The IAEA does not normally maintain stocks of reports in this series. However, microfiche copie f thesso e reportobtainee b n sca d from IN IS Clearinghouse International Atomic Energy Agency Wagramerstrasse 5 P.O. Box 100 A-1400 Vienna, Austria Orders shoul accompaniee db prepaymeny db f Austriao t n Schillings 100,- for e for e chequ a th f m th IAE mf o n o i n i r Aeo microfiche service coupons which may be ordered separately from the INIS Clearinghouse. FOREWORD Cancer incidenc increasins ei developen gi developin weln s i d a s la g countries. However, sinc somn ei e advanced countrie cure sth e rat increasins ei g faster tha cancee nth r incidence rate, the cancer mortality rate is no longer increasing in such countries. The increased cure rate ca attributee nb earlo dt y diagnosi improved san d therapy othee th rn handO . , until recently, in some parts of the world - particularly in developing countries - cancer control and therapy programmes have had relatively low priority. The reason is the great need to control communicable diseases. Toda yrapidla y increasing numbe thesf ro e disease undee sar r control.